What kind of compound would be least likely to dissolve in water?
1 Answer
Something that has extremely dissimilar intermolecular forces to those in water, especially if those forces are weaker than dipole-dipole interactions.
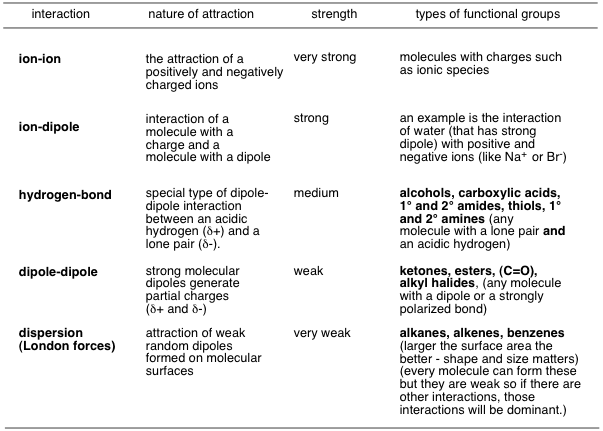
In general, we have a strength spectrum of intermolecular forces:
 http://www.chem.sc.edu/
http://www.chem.sc.edu/
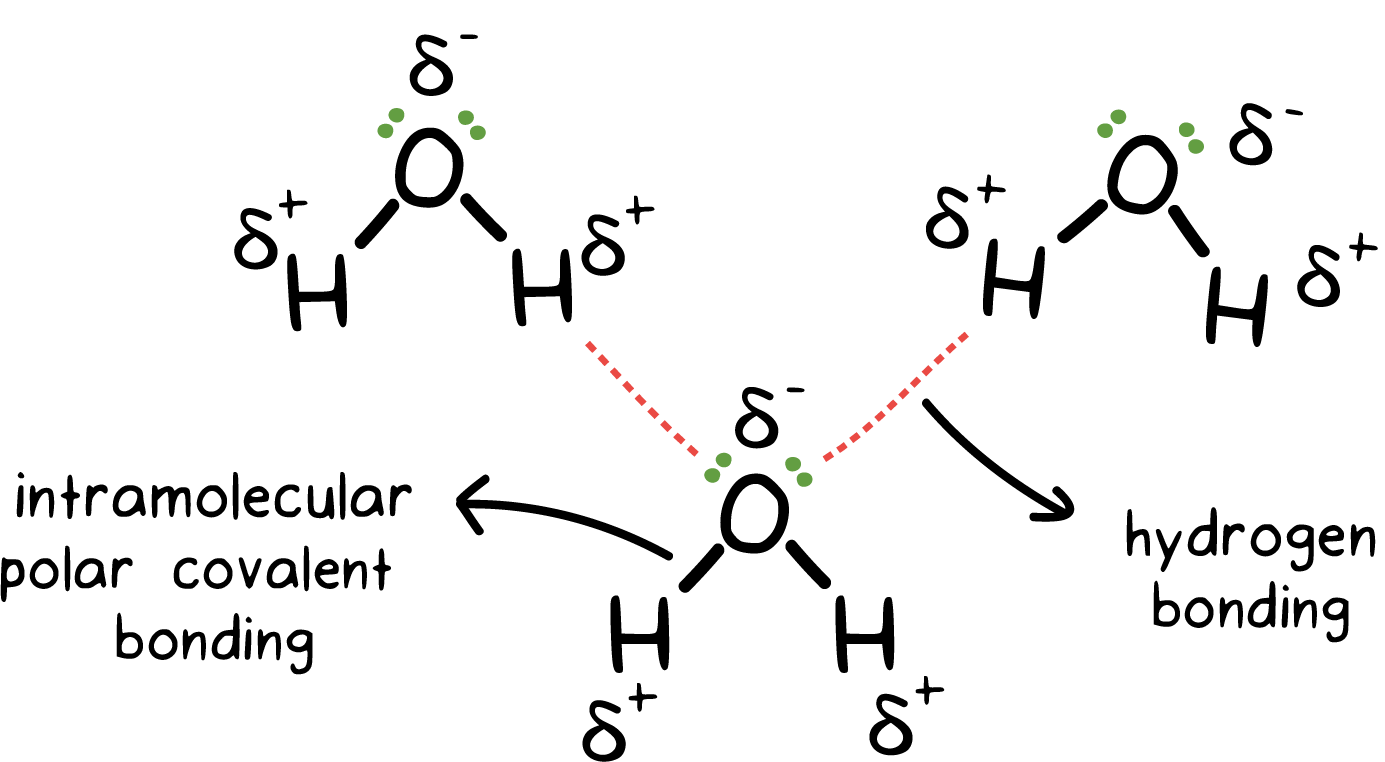
Water tends to have these intermolecular forces:
- London dispersion
- dipole-dipole
- hydrogen-bonding
 https://www.khanacademy.org/
https://www.khanacademy.org/
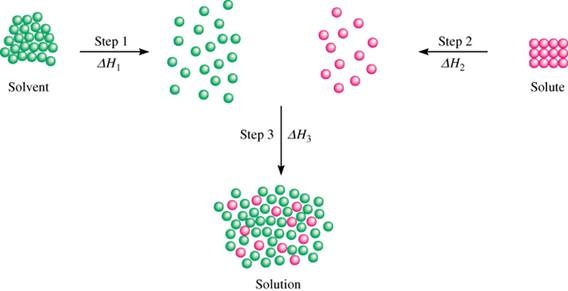
When a solute is not likely to dissolve in water, it means that...
The intermolecular forces in water are less able to break apart (so that water molecules can move apart, and the solute can squeeze in-between the water molecules) than the intermolecular forces in the solute are able to break apart.
 http://msjonasson.weebly.com/
http://msjonasson.weebly.com/
In other words, the energy trade-off for dissolving the solute in water is uneven; if it requires too much energy for the solute to dissolve, so it is unfavorable for it to dissolve.
(If
Since dispersion forces are weaker than hydrogen-bonding and dipole-dipole interactions, both of which are in water, it's not a good energy trade-off.
Therefore, solutes containing only London dispersion intermolecular forces dissolve most poorly in water.

