What number of unpaired electrons are there in Xe atom?
1 Answer
Zero.
Explanation:
We can start by writing the electron configuration of xenon,
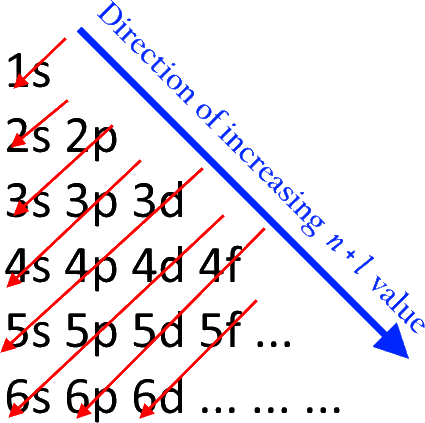 By Atchemey - Own work, CC BY-SA 4.0
By Atchemey - Own work, CC BY-SA 4.0
A neutral xenon atom has 54 electrons and would have the electron configuration
An electron is considered "unpaired" if it occupies a single atomic electron orbital with the other half of the orbital left unoccupied- possible only if the orbital is incomplete.
Each
Also, this fact is consistent with the observation that as a noble gas, xenon exists as a monoatomic gas at room temperature and is highly inert (chemically speaking). None of which are characteristic of a radical- an atom that has unpaired electrons.
Sources:
https://en.wikipedia.org/wiki/Xenon
 en.wikipedia.org
en.wikipedia.org
https://en.wikipedia.org/wiki/Unpaired_electron

