What product will we get from the reaction (i) C6H5CHO + CH3CH2OH (reagent HCl)→ (ii) CH3CH2C(Cl)2CHO + H20 (reagent H+) → ?
1 Answer
The products are probably i) benzaldehyde ethyl hemiacetal and ii) 2,2-dichlorobutanal hydrate.
i). Benzaldehyde + ethanol
This reaction appears to be a the formation of a hemiacetal.
Alcohols undergo acid-catalyzed addition to aldehydes to form hemiacetals.
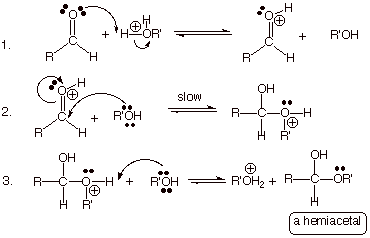
The reaction between benzaldehyde and ethanol is
ii) 2,2-Dichlorobutanal + water
Aldehydes undergo addition of water to form hydrates.
The reaction is catalyzed by acid and is greatly enhanced by electron withdrawing groups adjacent to the carbonyl group.
The

