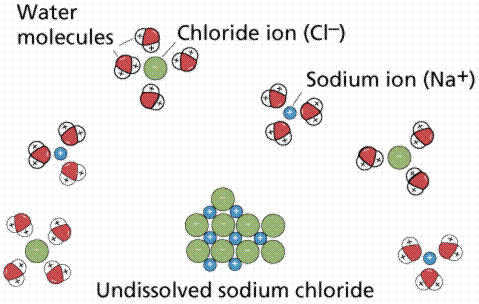
What structures are formed when water molecules surrounds individual ions?
1 Answer
May 22, 2018
These are in effect coordination complexes....
Explanation:
Water is an exceptionally good solvent for aquating ions...
And so when an ionic salt reacts in solution to give such ions we write….
And so when we write