What trend in electronegativity do you see as you go down a group/family on the periodic table?
1 Answer
See explanation
Explanation:
As you go across a period, electronegativity increases. As you go down a group, electronegativity decreases. Generally, electronegativity is highest in the upper right of the periodic table. Here's a visual:
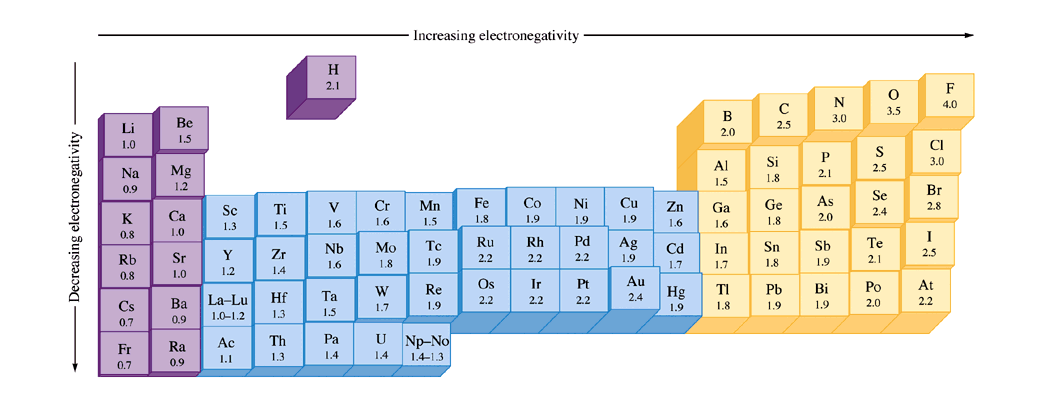
As you can see, in this version of the periodic table, the more the block pops out, the higher its electronegativity. You can also see the values underneath each element. The transition metals (in blue) are a little weird but I don't think it will matter much if you are taking general chemistry.
One neat trick I have is that Flourine, or

