What type of chemical bond are found between water molecules?
1 Answer
Sep 15, 2016
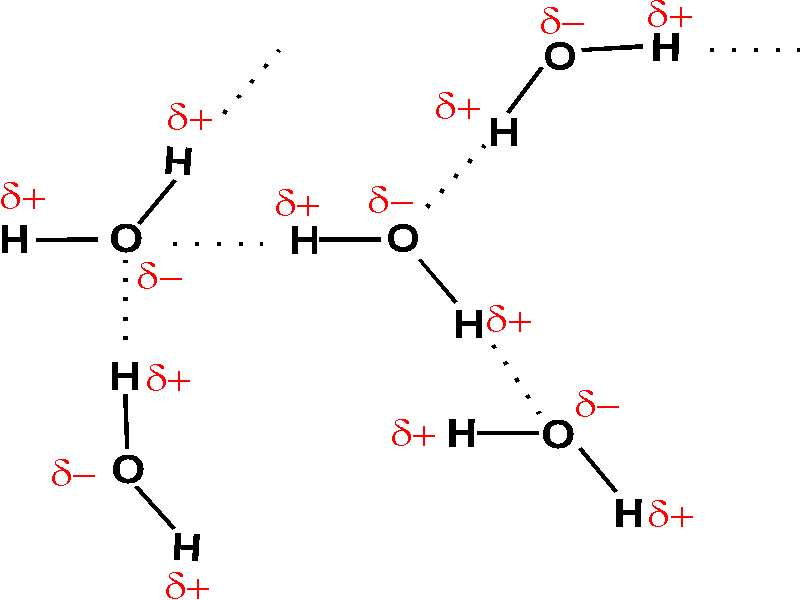
Between water molecules hydrogen bonds are found which are called as intermolecular hydrogen bonding
Explanation:
The water molecule has H-O-H, 2 hydrogen and one oxygen present. The O atom is highly electronegative in comparison to the H atom, hence it attracts the electron pair towards itself, making O atom itself partial negatively charged and H atom partial positively charged. So, water is a polar compound. The partially negative charged O atoms, (forms bond) attracts partially positively charged H atom from other molecule, forming hydrogen bond, and water forms intermolecular hydrogen bonding.