What type of reaction is #CH_3CH_3 + Cl_2 → CH_3CH_2Cl + HCl#?
1 Answer
This is actually a multi-step mechanism for a radical chlorination of ethane. You can always tell by knowing you are starting with an alkane and are reacting with a diatomic halogen. There are no other "standard" reactions like this.
OVERALL
#color(blue)("CH"_3"CH"_3 + "Cl"_2 stackrel(Delta, "or "hnu" ")(->) "CH"_3"CH"_2"Cl" + "HCl")#
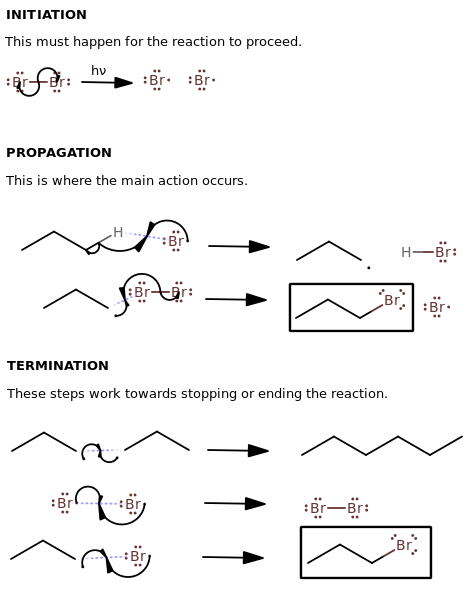
The reaction mechanism is shown below.
INITIATION STEP
#color(blue)("Cl"_2) stackrel(Delta, "or "hnu" ")(=>) 2"Cl"cdot#
PROPAGATION STEPS
#color(blue)("CH"_3"CH"_2"H") + cdot"Cl" => "CH"_3"CH"_2cdot + color(blue)("HCl")#
#"CH"_3"CH"_2cdot + color(blue)("Cl"_2) => color(blue)("CH"_3"CH"_2"Cl") + "Cl"cdot#
TERMINATION STEPS
#"H"_3"C"-"CH"_2cdot + cdot"H"_2"C"-"CH"_3 => "CH"_3"CH"_2"CH"_2"CH"_3#
#"Cl"cdot + cdot"Cl" => color(blue)("Cl"_2)#
#"CH"_3"CH"_2cdot + "Cl"cdot => color(blue)("CH"_3"CH"_2"Cl")#
Note that all of this occurs until the all reactant is consumed, so don't fret over the fact that what is shown here does not explicitly demonstrate conservation of mass.
You can see a more detailed version here, but for bromine with propane instead of chlorine with ethane.