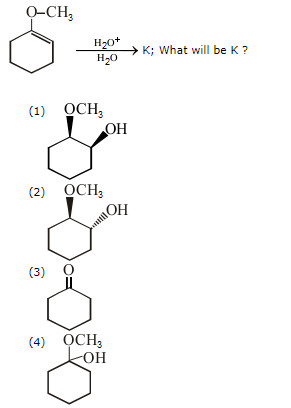
What will be the poduct of this reaction? Please show the mechanism.
1 Answer
Apr 6, 2018
This looks like an acid-catalyzed hydration of an alkene with a typo.
These proceed via carbocation formation (which seeks stability), and hence will generally exhibit Markovnikov regioselectivity.
 puu.sh
puu.sh
This would give a (roughly) racemic mixture of
Moroever, only water will deprotonate the oxonium ion because this is done in acidic conditions.

