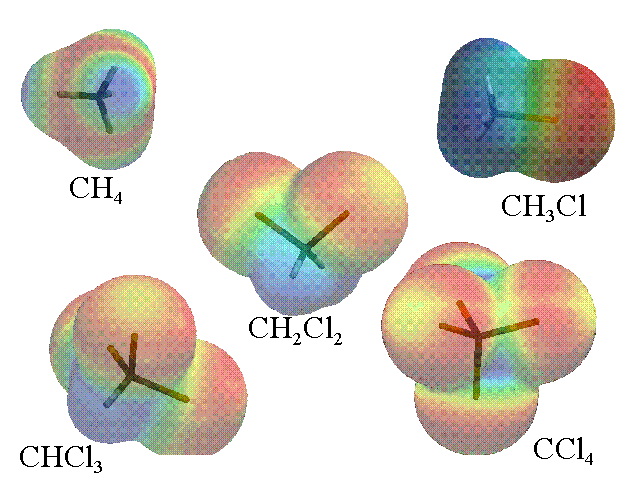Any 100% symmetrical tetrahedral molecule will be nonpolar.
Tetrahedral molecules have no nonbonding electron pairs and all identical bond angles. Therefore, the only way they can be asymmetric is if one atom is different from the rest.
When a symmetrical nonpolar molecule is made asymmetric by replacing a surrounding atom with a new atom, the nonpolar molecule becomes polar if the new atom is significantly different in electronegativity than the original atom.
Take #"CH"_4# and compare it to #"CH"_3"Cl"#.
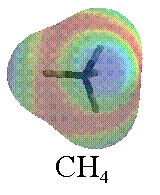
In #"CH"_4#, the electron density is slightly pulled inwards by carbon (carbon is more electronegative by about #0.4#), but each surrounding hydrogen gets that electron density pulled in a symmetrical, opposing direction.
So, #"CH"_4# is symmetrically polarized inwards, and thus every possible dipole moment vector cancels out and #"CH"_4# is not polar.
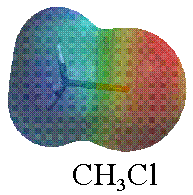
In contrast, #"CH"_3"Cl"# has a chlorine, which is more electronegative than carbon by about #0.6#. That means chlorine will pull on the electron density of carbon and polarize #"CH"_3"Cl"# towards chlorine (in #color(red)"red"#).
That asymmetrically polarizes #"CH"_3"Cl"# relative to the electron distribution of #"CH"_4#, thus making #"CH"_3"Cl"# polar.
You can compare the electron density maps for if you continue comparing with #"CH"_2"Cl"_2#, #"CHCl"_3#, and #"CCl"_4#.
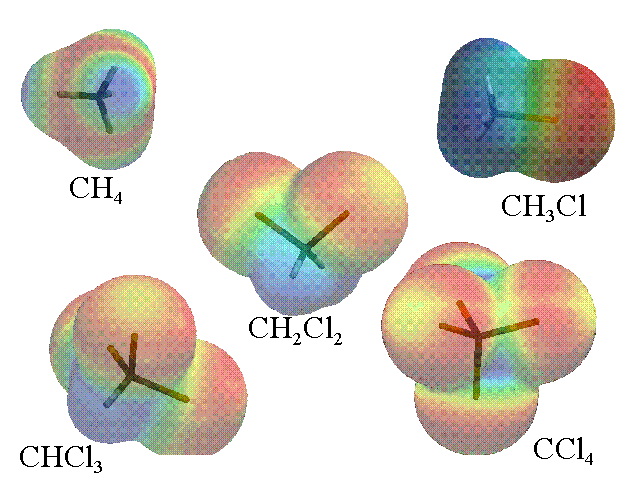
Note that when you compare #"CH"_4# and #"CCl"_4#, they are both symmetric, so they are both nonpolar.
However, the electron density distribution is more negative towards chlorine in #"CCl"_4# and more negative towards carbon in #"CH"_4#. Either way, it doesn't change the fact that symmetrical tetrahedral molecules are always nonpolar.