When is the cis-trans system not effective for naming geometric isomers?
1 Answer
It is easier to say when the cis/trans system is effective for naming geometric isomers.
There are only two cases in which the cis/trans system is effective.
- There are only two substituents of one type and two substituents of another type, with one member of each group on each end of the double bond.
Here, it is obvious which groups are cis or trans to each other.
Examples of this type of isomerism are the isomers of 1,2-dichloroethene

and of but-2-ene

- There are three different substituents, with an identical group on each end of the double bond.
Here, the pink groups are either on the same side or the opposite side of the double bond.
In this case, the system works best if the pink substituents represent carbon atoms in the main chain of the alkene and one of the other substituents is an H atom.
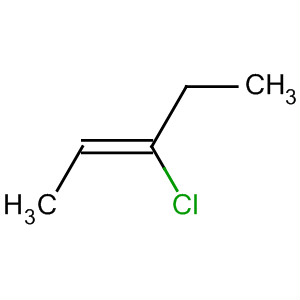
Examples are cis-2-chloropent-2-ene
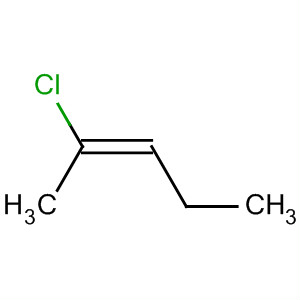
and trans-2-chloropent-2-ene.
We could give the compound below either of two names.
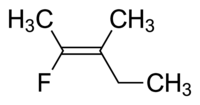
(a) cis-2-fluoro-3-methylpent-2-ene, because the two CH₃ groups are cis to each other, or
(b) trans-2-fluoro-3-methylpent-2-ene, because the methyl and ethyl groups that are part of the pentene chain are trans to each other.
To avoid this ambiguity, we should name such compounds by the E/Z system.

