Which elements have the highest electronegativities on the periodic table?
1 Answer
Jan 16, 2016
There isn't really a whole row that is the largest.
Explanation:
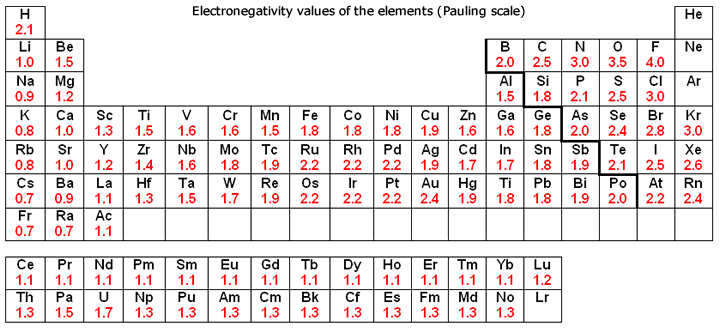
Elements from the halogen group including F, Cl, Br have pretty high electronegativities. The most electronegative element is Fluorine with a score of 4.0 (the highest possible.)
Across from Fluorine we also have N and O with high electronegativities.
Electronegativity is basically how much elements 'want' electrons. A simple way to think about it is that the closer an element is to Fluorine, the higher its electronegativity is.

