Which is more reactive towards nucleophilic attack, an aldehyde or a ketone? Give at least one reason why
1 Answer
Well, first let's look at the electrophilicity of that carbonyl carbon.
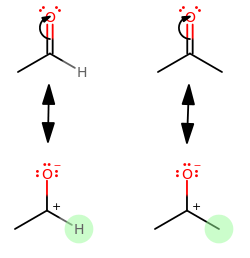
We should then notice that the difference is in the highlighted hydrogen vs. methyl group.
1) If we take away a proton from acetone, it would be taken away from a methyl, so we then get the following resonance structure for the conjugate base:
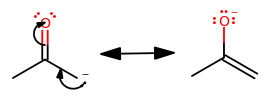
The point of this is not to show which one is more reactive in an enolate reaction, but to show that the methyl group is electron-donating to the carbonyl carbon.
Each methyl group makes the carbonyl carbon less electropositive, and thus less susceptible to nucleophilic attack.
Hydrogen, on the other hand, is (let's face it) out of electrons! It can't "donate" any electrons into the structure to make the carbonyl carbon less electropositive. It only had one, and it's used it. :P
So, using an aldehyde as a reference point, acetone is less reactive at the carbonyl carbon because it has TWO methyl groups, and acetaldehyde only has ONE.
2) A further argument you could say is that the methyl group is more of a steric hindrance than a mere proton. Obviously a four-atom substituent is larger than a single atom. And... yeah, that's the whole argument. Not quite as elaborate, but it contributes!

