Which of the following compounds exist, or do both of them exist? What are their names?
(a) #Hg(CNS)#
(b) #Hg(CNS)_2#
(a)
(b)
1 Answer
Mar 11, 2016
Explanation:
The compound given to you in option (b) exists and it's called mercury(II) thiocyanate.
It contains the mercury(II) cation,
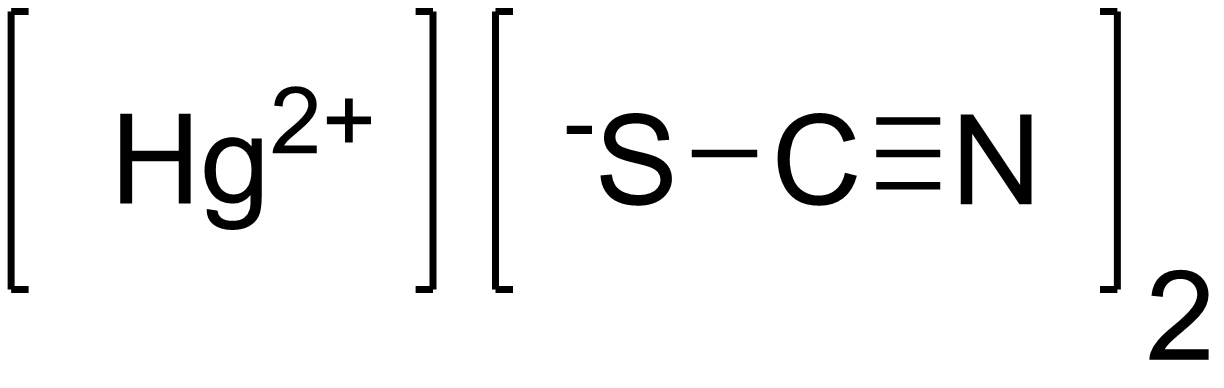
Mercury(II) thiocyanate is quite famous because its thermal decomposition causes an effect called the Pharaoh's Serpent.
Basically, the reaction, which is highly toxic, by the way, forms a brownish column that keeps growing as the reaction proceeds.
A detailed description of the decomposition reaction can be seen here

