Which of the following ions does not liberate hydrogen gas on reaction with dilute acids ?
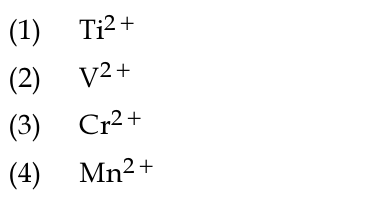
1 Answer
Mar 5, 2018
Explanation:
These are transition metals and reactivity decreases as we move from
