Which of the following polyatomic ions has a 3- ionic charge: hydrogen carbonate, hydroxide, sulfate, nitrate, or phosphate?
1 Answer
Nov 3, 2015
Explanation:
You can always calculate for the formal charges, or better yet, draw the structure then count the number of electrons. But if you find these methods taxing, then you can always memorize the list of anions and their charges.
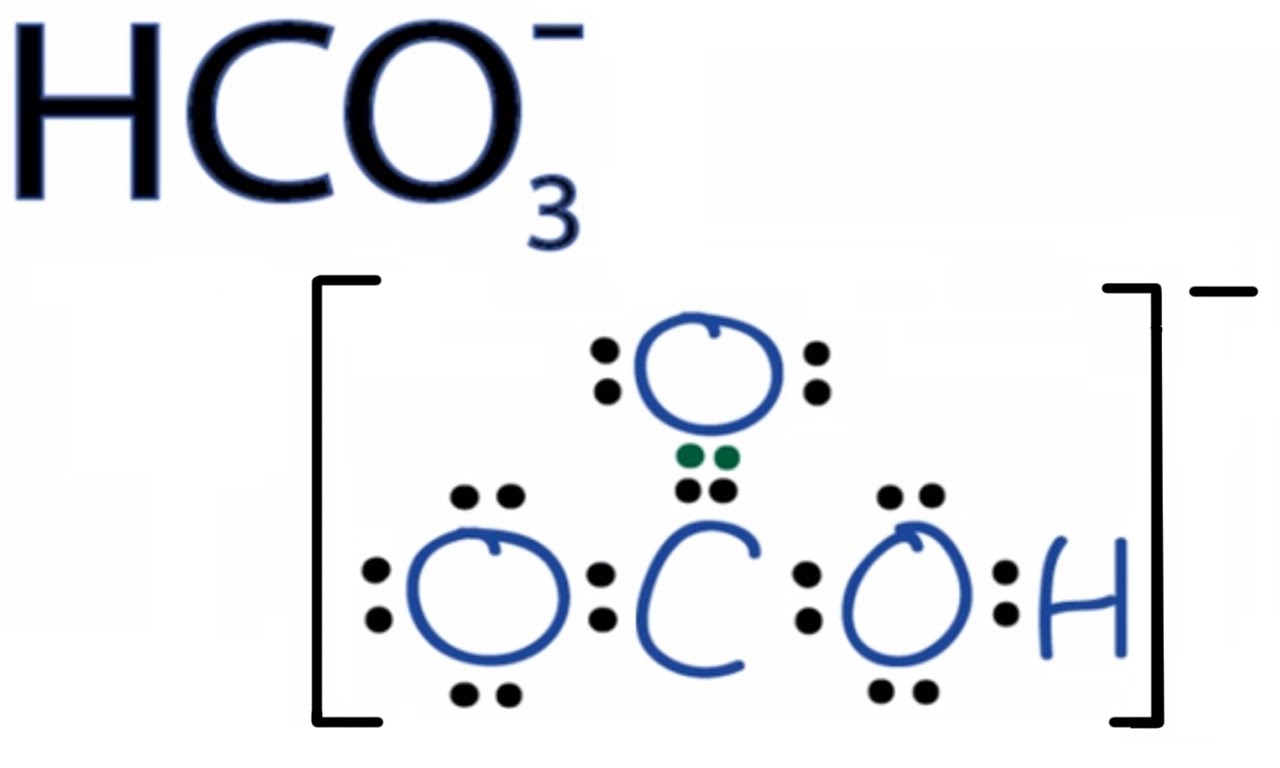
(1) hydrogen carbonate or bicarbonate ion:
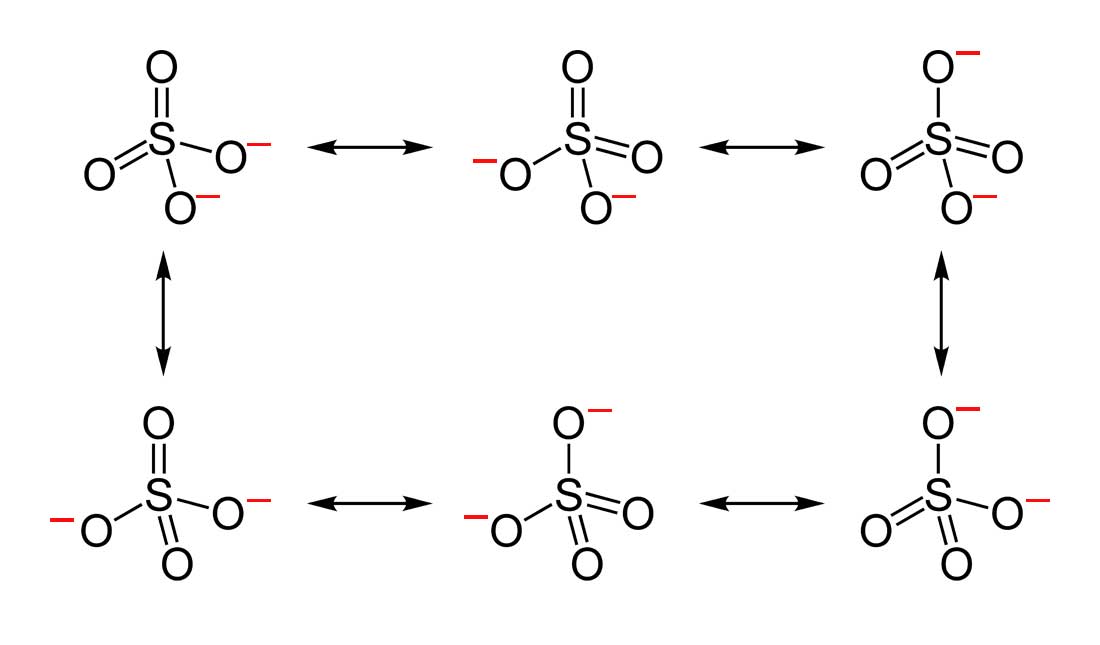
(2) sulfate ion:
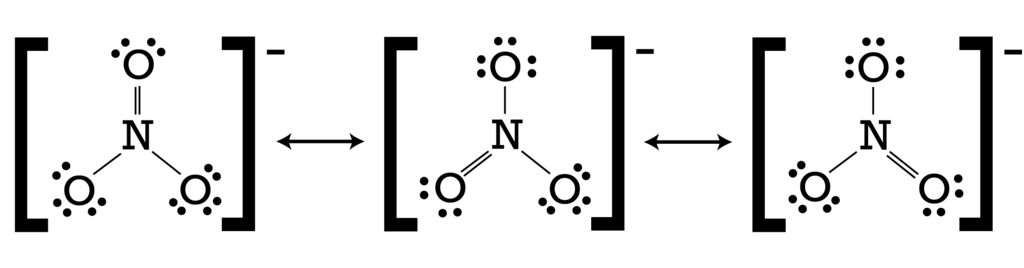
(3) nitrate ion:
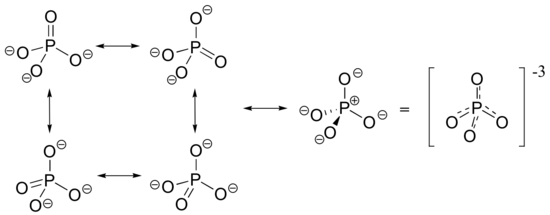
(4) phosphate ion: