Which of the following would have a noble gas configuration; Na+2, O−3, Cl−1, or Ca+3? Why?
1 Answer
The chloride ion,
Explanation:
All of the ions except
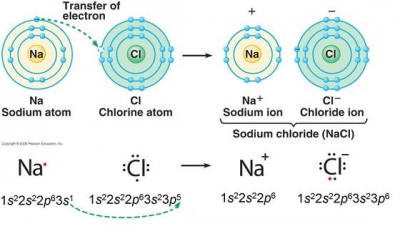
The element chlorine has atomic number 17, which is the number of protons in the nuclei of its atoms, and a neutral atom of chlorine will also have 17 electrons.
A neutral chlorine atom has seven valence electrons in its valence shell. It needs one more electron to become stable by filling its valence shell, which requires eight electrons. This is called the octet (8) rule.
In order to do this, a chlorine atom gains one electron from another atom, primarily an atom of a metallic element. By gaining one electron, it now has an octet, but also now has one more electron than protons, so it has a
 https://dashboard.dublinschools.net/lessons/?id=40c7761350a00d1c84d2231dfd884341&v=2
https://dashboard.dublinschools.net/lessons/?id=40c7761350a00d1c84d2231dfd884341&v=2

