Which option is correct ..??
In the fraction distillation of petroleum...
A. the hydrocarbons in each fraction have similar boiling points.
B. the fraction with higher boiling points are collected high in the fractionating columns.
C. the fractions are separated according to their melting points.
D. the fractions collected at the to are alkanes, followed by the alkenes.
In the fraction distillation of petroleum...
A. the hydrocarbons in each fraction have similar boiling points.
B. the fraction with higher boiling points are collected high in the fractionating columns.
C. the fractions are separated according to their melting points.
D. the fractions collected at the to are alkanes, followed by the alkenes.
1 Answer
A
Explanation:
The process of Fractional distillation is used to separate more than one liquid from a solution. The process makes use of the difference in boiling points of constituent liquids.
When we heat a mixture of liquids which have different boiling points, the liquid with lowest boiling point starts to boil first and vaporizes. During the process of vaporization the liquid uses the supplied heat for change of its state while the temperature of mixture remains constant till all the liquid fraction has evaporated. This heat is called latent heat of vaporization or evaporation. The vapor thus produced is then condensed and convert back to liquid.
Crude oil is separated into its fractions by fractional distillation as shown below. The fractions at the top of the fractionating column have lower boiling points than the fractions at the bottom.
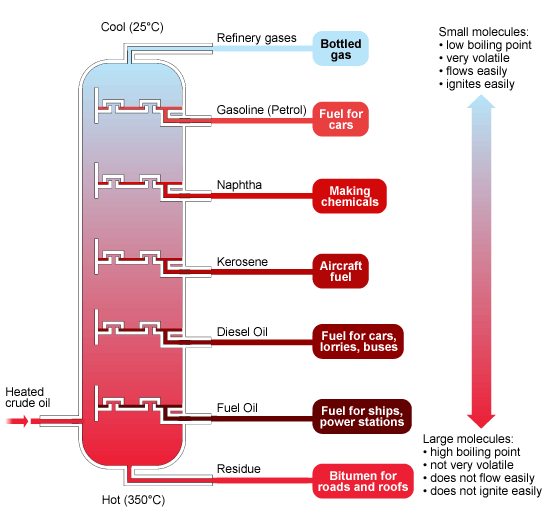 http://www.bbc.co.uk
http://www.bbc.co.uk
The gases condense at the top of the column, the liquids in the middle and the solids stay at the bottom.
