#s# orbitals; those have the most radial nodes (where the wave function goes to zero for a given #r#) out of the orbital shapes of the same #n#, so there are less places electrons can be, which expands the orbital radius to allow electrons to reach in farther.
This can be seen in the radial density distributions:
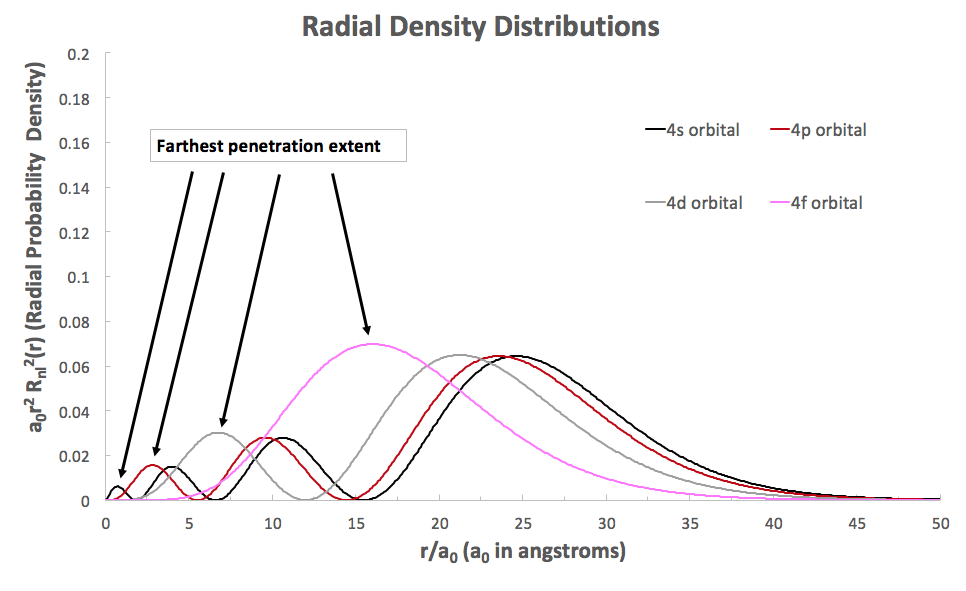
These graphs plot the time-averaged position of electron density as one increases the viewing window we have of a given hydrogen atomic orbital.
We should see here that #bbs# orbitals reach in the farthest, and it can be determined from the "farthest penetration extent" pointed out above.
The #f# orbital of the same #n# is the least penetrating, as it tapers off at around #8# bohr radii, whereas the #s# orbital of the same #n# tapers off at less than #1# bohr radius from the nucleus (#r = 0#).


