Which pair of bonds is more polar: P-Br and P-Cl?
1 Answer
The
Explanation:
A polar bond is basically a bond where electrons are more drawn to one atom over the other atom.
That one atom will have a greater electronegativity, which means that it has a greater tendency to hog electrons in a bond.
When comparing two different polar bonds—two bonds that already have an uneven distribution of electrons—the one with the greater difference in electronegativity will be more polar.
This is because, as the electronegativity difference gets greater, electrons will spend more and more amounts of time with that more electronegative atom.
So, with that being said, here's the positions of
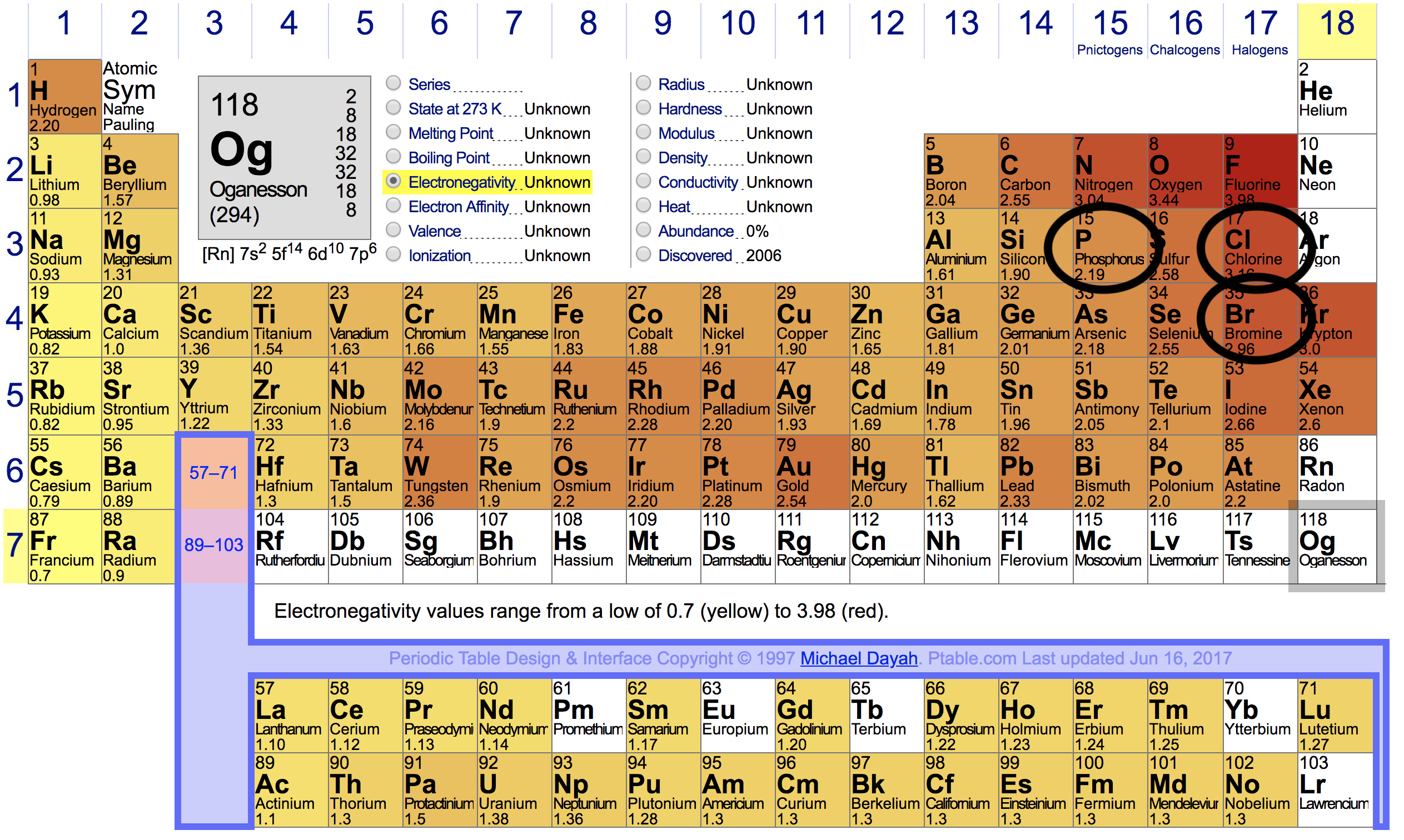
Electronegativity differences are:
#3.16-2.19=0.97# for a#P–Cl# bond.#2.96-2.19=0.77# for a#P–Br# bond.
So, the

