Why are aromatic rings stable?
1 Answer
Aromatic rings are stable because they are cyclic, conjugated molecules.
Explanation:
Conjugation of π orbitals lowers the energy of a molecule.
Thus, the order of stability is ethene < buta-1,3-diene < hexa-1,3,5-triene ≪ benzene.
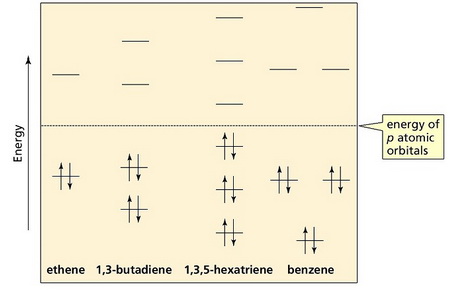
(From Kshitij IIT JEE Online Coaching)
Benzene is different because it is a cyclic conjugated molecule.
In a cyclic conjugated molecule, each energy level above the first occurs in pairs.
Thus, the total energy of the six π electrons in benzene is much less than the energy of three separate ethene molecules or even of a hexa-1,3,5-triene molecule.
A Frost circle reflects the pattern of orbital energies.

You place the ring inside a circle with one of its vertices pointing downwards.
Then you draw a horizontal line though the vertices. This represents the orbital energy levels.
If there isn't one already, you draw a horizontal dashed line through the circle's (and molecule's) centre. This represents the nonbonding energy level.

Qualitatively, a Frost circle explains the
You need 2 electrons to fill the first level and 4 electrons to fill each level above the first.

