Why are the electronegativity values of noble gases zero?
1 Answer
Because when Pauling defined his electronegativity scale, he had never witnessed
If you want to see the history behind this, and the derivation of the equations he used, scroll down to the very bottom of the answer.
Linus Pauling defined his electronegativity scale (which most general chemistry classes will use) in a way that required knowing experimental bond enthalpies of actual compounds.
He specified that fluorine's electronegativity was arbitrarily
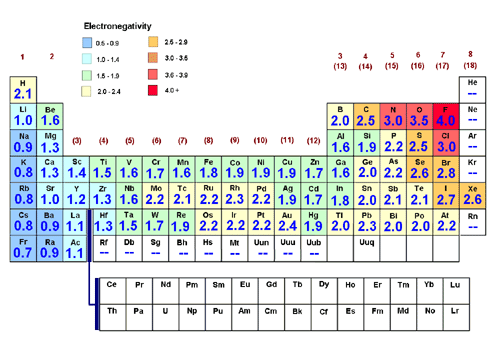
These calculations specifically required that to know the electronegativity of element
- The electronegativity of element
#"A"# - The bond enthalpy of an
#"B"-"B"# bond - The bond enthalpy of an
#"A"-"A"# bond - The bond enthalpy of an
#"A"-"B"# bond
At the time, he had not witnessed
For all noble gases at the time, Pauling couldn't have known:
- The bond enthalpy of an
#"A"-"B"# bond - The bond enthalpy of an
#"B"-"B"# bond
Thus, he couldn't find values for them.
So really, it's more accurate to say that the noble gases, under the Pauling definition of electronegativity, mostly have undefined electronegativity values.
NOTE: However...
Apparently, there is a
Based on that,
NOTE: Also...
Apparently,
- The
#"Xe"_2# dimer has been made before. - Like I said,
#"XeF"_6# exists (as well as#"XeF"_2# and#"XeF"_4# ).
So, it was possible to find that electronegativity as well.
NOTE: Oh yes, and...
And you know what,
APPENDIX BELOW!
DISCLAIMER: You do not need to know how to calculate the electronegativities of atoms, because Linus Pauling already did. You should just know how to use electronegativities.
Here's how Linus Pauling came up with the electronegativities in 1932. Coming from a Physical Chemistry background, I have a pretty good understanding of what's being discussed here.
I will list the equation near the bottom, just so you can know that there was indeed an equation. But you don't have to know how to use it.
REPRESENTING A MOLECULE
Pauling starts from the wave function (a physical chemistry concept), which, in this particular scenario, describes the interaction of two atoms to make a molecule from the standpoint of quantum mechanics.
He begins with a linear combination of averaged states:
#Psi_(AB) = c_1phi_(AB) + c_2phi_(A^(+)B^(-)) + c_3phi_(B^(+)A^(-))# where:
#Psi# is a time-dependent wave function, which basically represents a molecule in this case.#phi# is a "trial function", which just means it's a function Pauling got from trial and error and used to solve the wave function. In other words, it's a function he figured out after fidgeting a lot with numbers.#c_1# ,#c_2# , and#c_3# are expansion coefficients, correlating with the linear combination of atomic orbitals (LCAO) concept. They are a direct experimental result of this linear combination, and they aren't important to us, specifically.
IONIC/COVALENT CONTRIBUTIONS TO THE CHEMICAL BOND
Thus, the functions after the equal sign, in order from left to right, represent the covalent character
#"*"# Covalent character: the component that describes a pure covalent bond.
#"*"# Ionic character: each of the two components denotes how it might theoretically be if all electrons belonged to only one of the two contributors in the bond (#A# or#B# only).
The idea with these components is that they basically account for the fact that not all bonds are
In other words, if for example,
This corresponds with the idea of electronegativity, since it implies the potential for unequal sharing of electrons towards the more electronegative atom, which correlates with what we today say electronegativity differences explain.
DEVIATION FROM PERFECTLY EQUAL ELECTRON SHARING
When Pauling proceeds to determine a relationship for calculating electronegativity, he starts with this equation:
#Delta' = D_(AB) - sqrt(D_(A A) D_(BB))# where:
#Delta'# is the "additional ionic character", which describes the degree/amount/extent of polarization of charge towards one member of the bond or the other.#D# is the experimentally-measured bond energy.#AB# is a label for two different bonding members. Thus, it stands for the hypothetical interaction of THE two investigated bonding members whose electronegativity differences are unknown.#A A# is a label for two identical#A# bonding members, and#BB# for two identical#B# bonding members. Thus, it stands for the hypothetical interactions of two bonding members whose electronegativity differences are#0# .
Hence, that basically says that the amount of polarization towards one bonding member versus the other is somehow dependent on the difference in energy between bonding the two different atoms and bonding two identical atoms.
THESE DEVIATIONS CORRELATE WITH OUR CONCEPT OF ELECTRONEGATIVITY DIFFERENCES
Pauling then postulates that this additional ionic character
#Delta' = f("EN"_A - "EN"_B)#
And after fudging with some numbers, he determines that the function is:
#Delta' = 30("EN"_A - "EN"_B)#
OVERALL ELECTRONEGATIVITY EQUATION
Thus, the equation he figures out is:
#color(blue)(D_(AB) = sqrt(D_(A A) D_(BB)) + 30("EN"_A - "EN"_B))#
Overall, what Pauling had to know were the
Then, since Pauling didn't know the electronegativities yet, he assigned

