why are the energies below different ? both are just breaking the same type of bond: ONCl --> NO + Cl >H= 158 kj mol-1 NCl3 --> NCl2 + Cl >H= 375 kj mol-1
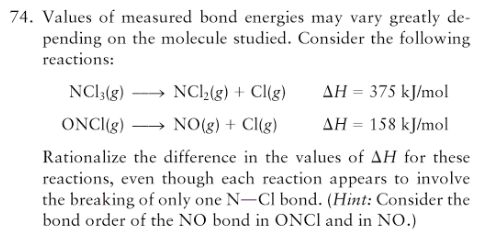
1 Answer
Feb 9, 2018
Well, look at the Lewis structures.
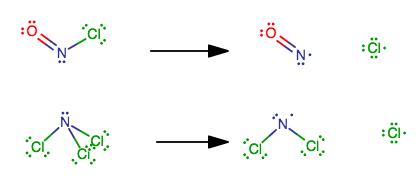
The
That is seen as an

