Why are the lone pairs in the diagram below connected with dashed lines and triangular shapes? Do they signify anything?
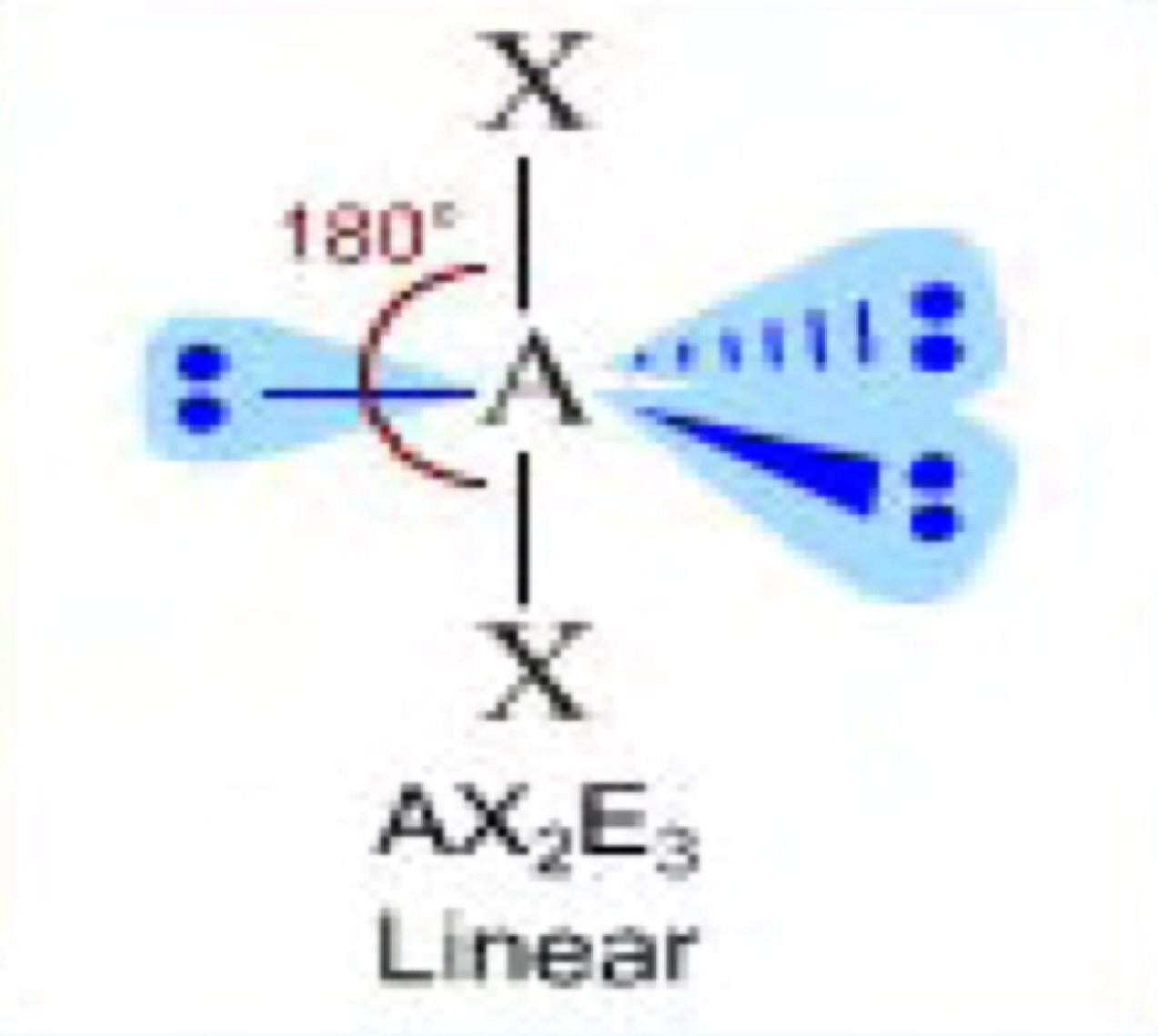
I understand that the three electron pairs will repel each other to form a linear bond but then why does the diagram include triangles as an indication for a bond? (They are in blue)
If anyone could help, I'd appreciate it. Thx:-)

I understand that the three electron pairs will repel each other to form a linear bond but then why does the diagram include triangles as an indication for a bond? (They are in blue)
If anyone could help, I'd appreciate it. Thx:-)
1 Answer
Here's what's going on here.
Explanation:
The
The idea here is that you have
regular lines
#-># they are located in the plane of the pagedashes
#-># they are directed behind the pagewedges
#-># they are coming out of the page
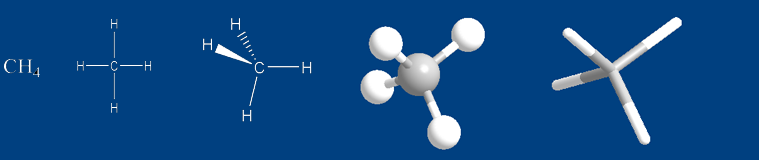
In your case, you have two single bonds to
The lone pair placed on the dash is being drawn going away from you, behind the plane of the page.
The lone pair placed on the wedge is being drawn coming at you, in front of the plane of the page.


