Why binding energy (Mettalic bond ) of Mn is less than Cr??
1 Answer
If you mean nuclear binding energy, then that's not true.
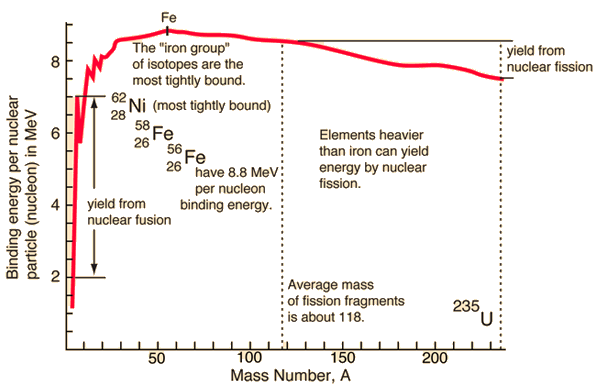
From this graph, with mass numbers of
When I hear binding energy, I think of the photoelectric effect, i.e. the energy input required to remove an electron from the metal, or the work function, or the threshold energy
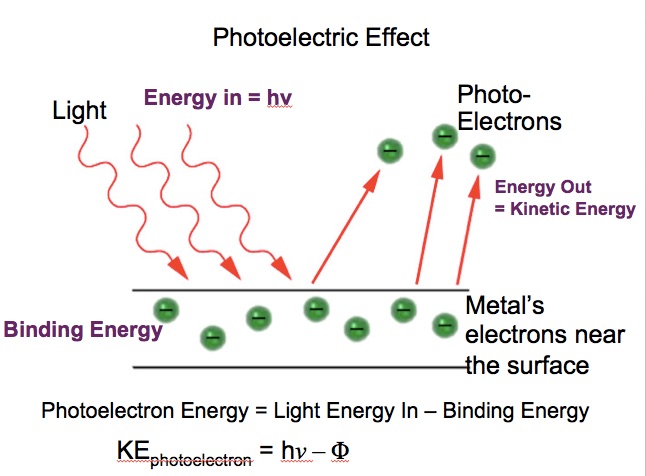
In that case,
Since the binding energy is less for
#underbrace(ul(uarr color(white)(darr)))_(Cr)# #" "" "# #underbrace(ul(uarr darr))_(Mn)#
Hence, there is electron repulsion among the

