Why chelate compounds are stable than nonchelated compounds?
1 Answer
Well, to compare them on equal footing, we would be considering the same bonds being made with each kind of compound.
Consider the following reaction:
#["M"("NH"_3)_6]^(z+) + 3(en) rightleftharpoons ["M"(en)_3]^(z+) + 6"NH"_3#
where
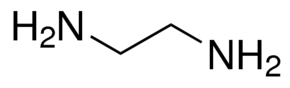
Visually, at first glance, the only difference between
(They both have the same atoms that bind, and they both contribute zero charge.)
This will therefore have a net zero enthalpy for the breaking and formation of the six
All that means is that enthalpy is not a significant stabilizing factor.
The main difference we're looking for is that
Entropy is smaller with more restricted motion.
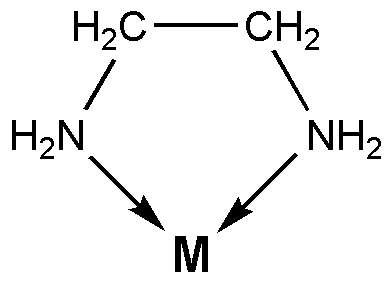
Therefore, its entropy of bond formation is less positive, and the decrease in entropy due to the interaction corresponds to favoring
We call this the chelate effect, where the binding of a chelating ligand like

