Why dipole moment of cyclopentanone is greater than cyclopentadienone?please help me.
1 Answer
Jan 9, 2017
Here is my explanation.
Explanation:
Cyclopentanone
In addition to the bond dipole caused by the C-O electronegativity difference, there is also a resonance contributor
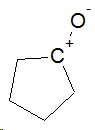 Cyclopentanone
Cyclopentanone
The combination of the two effects gives cyclopentanone a dipole moment of 3.28 D.
Cyclopentadienone
The corresponding resonance contributor in cyclopentadienone is
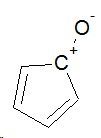 Cyclopentadienone
Cyclopentadienone
In this case, generating a positive charge on the carbonyl carbon generates a highly unstable antiaromatic 4π system.
The relative importance of this contributor will therefore be less, and the dipole moment will be decreased (to 3.13 D).

