Why do 0.60 grams of acetic acid dissolve in 200 grams of benzene to form a solution that lowers the freezing point of benzene to 5.40°C?
1 Answer
The freezing point is lowered because adding the acetic acid disrupts the dynamic equilibrium of the freezing process.
There is a dynamic equilibrium at the freezing point of benzene. Molecules of benzene leave the surface of the solid at the same rate as they return.
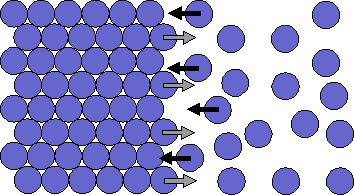
Adding acetic acid to the system will disrupt the equilibrium. The acetic acid molecules dissolve in the benzene, but they do not pack easily into the array of molecules in the solid.
Also, there are fewer benzene molecules on the liquid side because acetic acid has replaced some of the benzene molecules. The total number of benzene molecules captured by the solid per second goes down, so the rate of freezing goes down.
The presence of the acetic acid does not change the rate of melting, so melting occurs faster than freezing.
To re-establish equilibrium, you must cool the acetic acid-benzene mixture to below the normal freezing point of benzene.
You can learn how to calculate the freezing point depression at
http://socratic.org/questions/how-do-you-calculate-freezing-point-depression
In your problem,
For benzene,
Hope this helps.

