Why do geometric isomers exist in some alkenes?
1 Answer
May 19, 2018
Because there is no possibility of free rotation around an olefinic bond...
Explanation:
Consider
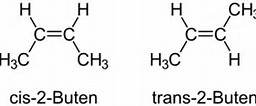
For BOTH isomers, the
Such isomerism is also possible for disubstituted rings..and given

