Why were noble gases said to have no electronegativity?
1 Answer
Jun 15, 2017
Noble gases do have an electronegativity. Pauling just didn't have them at first because one thing he needed was homonuclear bond energies.
Explanation:
All the noble gases except helium and neon form compounds with highly electronegative elements.
Chemists have been able to calculate their electronegativities as
The calculated electronegativity of
Here's a Periodic Table that includes the electronegativities of the noble gases.
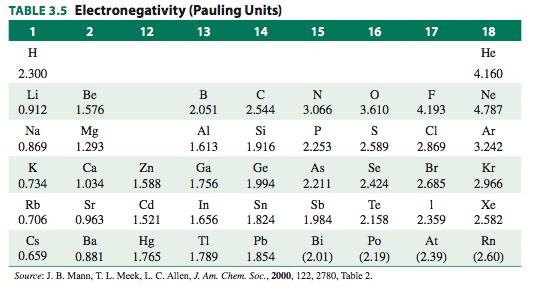
(Adapted from Inorganic Chemistry, Miessler et al., 5th ed., pg. 58)


