Why do solutes lower the freezing point?
1 Answer
Nonvolatile solutes lower the freezing point by blocking the solvent particles from congregating.
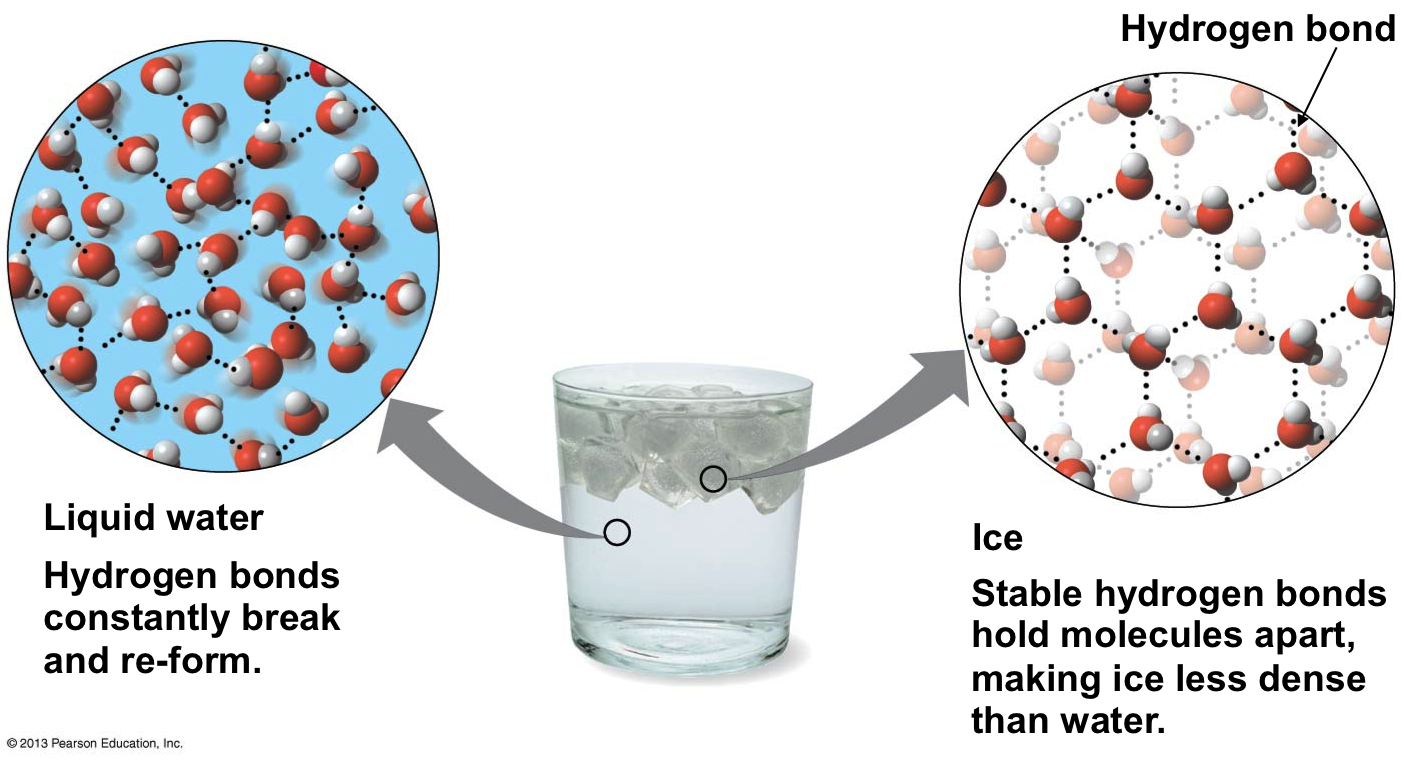
When freezing occurs, solvent particles have to come together and form a crystal structure with stable intermolecular forces, and that requires a specific interaction distance since the particles cannot do more than vibrate once they are fixed in place.
Here is water freezing normally:
Solutes you add into the solvent will block the solvent from doing so:
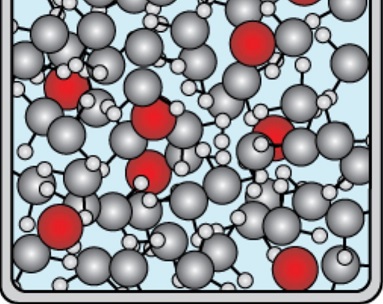
And thus, nonvolatile solutes make it harder to freeze, lowering the freezing point.
The same solutes will also raise the boiling point.
The same concept applies... they block the solvent from leaving the surface of the solution, and hence limit vaporization. That makes it harder to boil and thus raises the boiling point.

