Why does beta decay occur?
1 Answer
Beta decay represents the transformation of a neutron from the nucleus of a radioactive element into a proton, an electron, also called a beta particel, and an antineutrino.
The electron and the antineutrino are emitted from the nuclues, which now has one extra proton; this essentially changes the element, since the atomic number has now increased by 1.
There are two types of beta decay, beta-minus, which I described in the previous paragraph, and beta-plus, in which a proton decays into a neutron, a positron, and a neutrino.
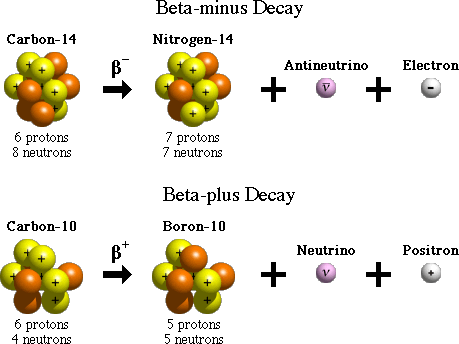
Both reactions occur when an atom has either too many protons, or too many neutron in its nucleus. One type of beta decay or the other will move the atom closer to the region of stability in the Chart of Nuclides, essentially bringing the atom closer to the optimal ratio between protons and neutrons.
The Chart of Nuclides represents a two-dimensional graph which shows an atom's number of protons on one axis ,and the number of neutrons on the other. This system provides a great insight into the characteristics of isotopes.
Here's what this chart looks like:
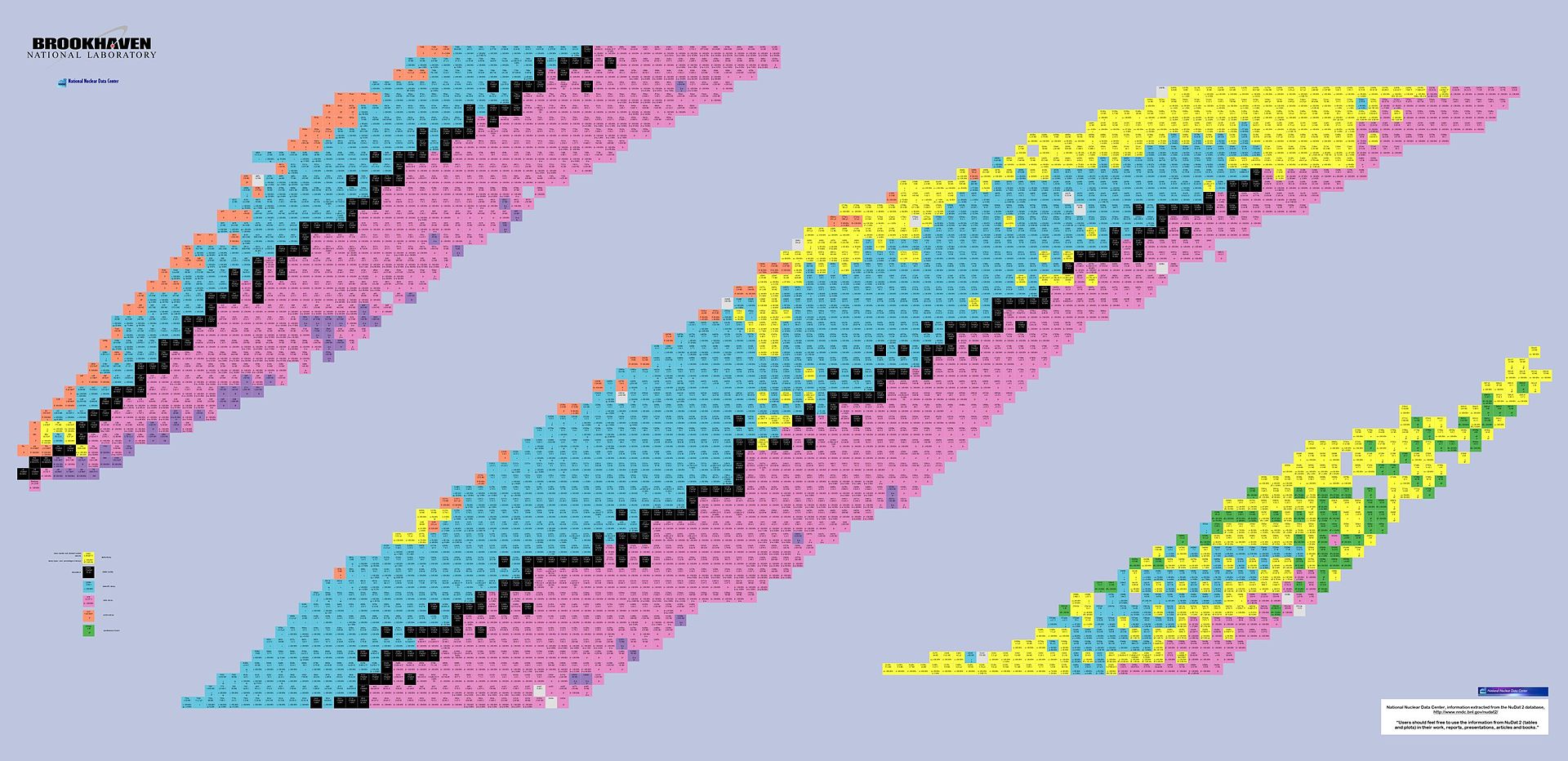
The stable atoms are shown in black, while those that undergo beta decay are shown in light blue.
Here's an interactive chart that offers more detail:

