Why does E-Z isomerism occur?
1 Answer
Jun 11, 2015
Explanation:
In
- restricted rotation, often involving a
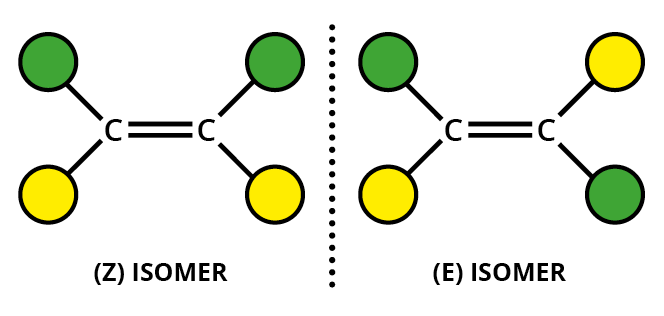
C=C double bond - two different groups on one end of the bond and two different groups on the other end.
For example, an alkene such as but-2-ene has two different groups on each alkene carbon.
It can exist as
 www.4college.co.uk
www.4college.co.uk
The substituents can be given "priorities", with atoms with higher atomic numbers given higher priorities (the Cahn-Ingold-Prelog rules).
If the highest priority groups for each carbon are on the same side of the molecule, we have the
If the highest priority groups for each carbon are on opposite sides of the molecule, we have the
 www.compoundchem.com
www.compoundchem.com

