Why does fluid with high viscosity have strong attractive forces and greater internal friction?
1 Answer
Aug 4, 2017
Let's not reverse the cause and effect here. It is because the fluid has stronger attractive forces and greater internal friction than fluids with lower viscosity that they have higher viscosity...
And that is due to stronger intermolecular forces. The more polar solvent molecules interact more strongly, and thus, solvent flow becomes more difficult because interactions keep forming reforming as the molecules move past each other.
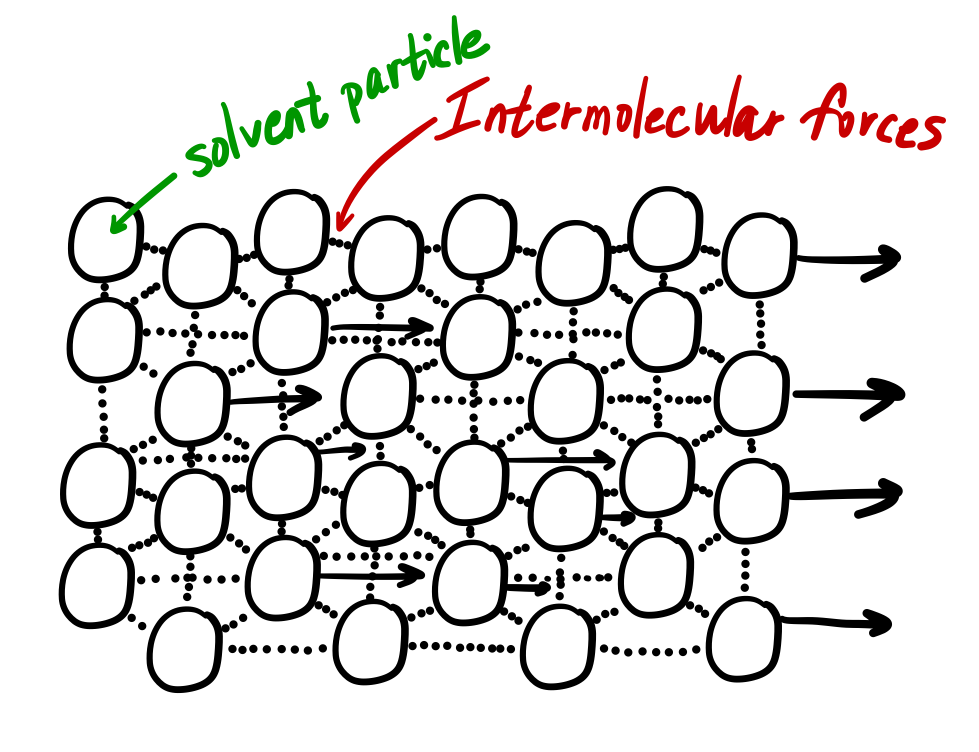
(I couldn't find an image for this, so I had to draw it myself.)

