Why does fluorine have a higher ionization energy than bromine?
1 Answer
See the explanation.
Explanation:
Ionization energy is the energy required to remove a valence electron from an atom or ion in its gaseous state, causing it to gain a positive charge as the electron is removed.
Fluorine is in period two of the periodic table, meaning that it contains electrons in only the first two energy levels at ground state. It has a higher ionization energy than bromine due to the fact that the valence electrons of fluorine are attracted more strongly to its positively charged atomic nuclei. This is because fluorine atoms have only two energy levels, so there is no significant shielding effect . Therefore it requires a higher ionization energy to remove a valence electron from fluorine.
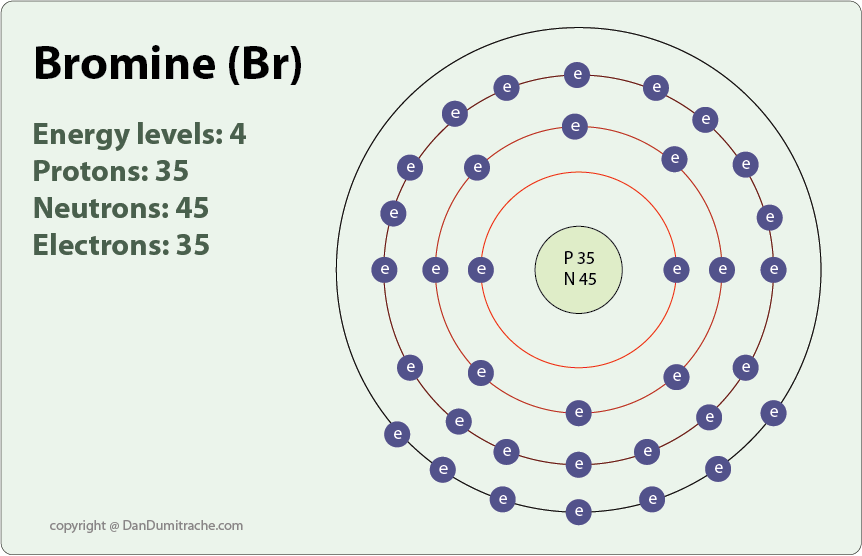
Bromine is in period four of the periodic table. In its ground state, it has electrons in the first four energy levels. There are three energy levels of electrons to shield the valence electrons from the positively charged atomic nuclei. This means that bromine holds its valence electrons more loosely, requiring a lower ionization energy than fluorine to remove a valence electron.