Why does radiation transfer heat energy?
1 Answer
Because it is a wave.
Explanation:
Infrared radiation (heat) is a form of electromagnetic wave.
Waves are a method of energy transfer that do not require a medium (e.g. vibrating atoms).
Therefore, as radiation is a wave, it can transfer energy. In fact, it doesn't just transfer heat energy. Visible light is just another form of EM radiation.
If an object is heated, it gains energy. What we mean by this is that the individual atoms making up the object gain energy. However, these atoms will also emit energy in the form of electromagnetic waves.
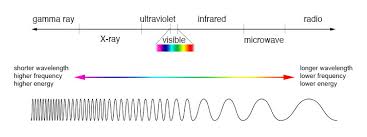
It is worth noting that (in general), as an object gets hotter, it will emit shorter waves with a higher frequency. This is as the shorter a wavelength is, the more energy it has - and if an object is very hot, then it will have more energy.
I hope this helped; let me know if I can do anything else - waves and radiation can get confusing!!
