Why does shielding effect increase down a group?
1 Answer
Shielding increases DOWN a Group because the nuclear core is farther removed from the valence electrons.
Explanation:
Complete electron shells shield the nuclear charge very effectively. The best way to appreciate this is to consider the atomic radius, period by period. Across the Period, from left to right, the atomic radius progressively decreases. The nitrogen atom is larger than the oxygen, which is larger than the fluorine atom, which is larger than the neon atom. (You should perhaps look at actual metrics listing atomic radii).
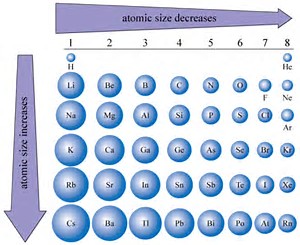
As we descend a Group, a column on the Periodic Table, electrons add to a new shell, which is (i) farther removed from the nuclear core, and (ii) which is effectively shielded from the nucleus by the interposing electronic shells. The result is that atomic radii increase, and ionization energies (another way to interrogate the phenomenon) DECREASE.

