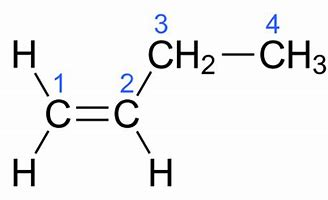
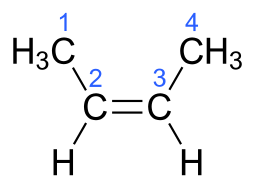We gots #"1-butylene"#, #H_2stackrel1C=stackrel2Cstackrel3CH_2stackrel4CH_3#...
versus #"2-butylene"#, #H_3stackrel1Cstackrel2C(H)=stackrel3C(H)stackrel4CH_3#...
But #"2-butylene"# can generate geometric isomers, the which retain the SAME connectivity but a different geometry, viz. #"cis"# and #"trans"# isomers...
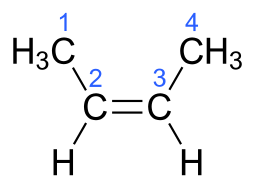
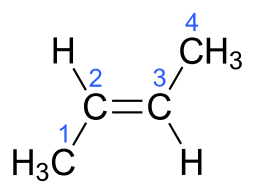
In each case, the carbon-carbon connectivity is MANIFESTLY the same i.e. #C1# connects to #C2#....to #C4#...but the geometry is manifestly different. And this gives rise to different physical properties, and chemical properties...i.e. the trans isomer has a boiling point of #0.9# #""^@C#, whereas the cis isomer has a boiling point of #3.7# #""^@C#.
That the trans isomer is MORE volatile than the cis isomer is an unusual phenomenon.
On the other hand, for #"1-butylene"# ONLY the one isomer is conceivable...