Why is ethylene planar?
1 Answer
Oct 8, 2016
There is no rotation about the axis joining the 2 carbon atoms, therefore ethylene is a planar molecule.
Explanation:
The diagram below shows the bonding of ethylene molecule.
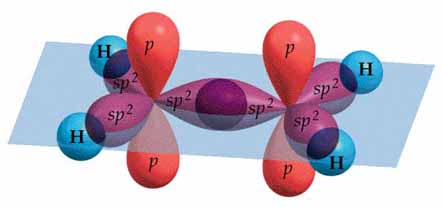
The
The molecule cannot be twisted without breaking the

