Why is helium not the most electronegative elemnt?
3 Answers
It has no electronegativity because it is a noble gas.
Explanation:
Electronegativity describes an atoms relative tendency to attract a pair of electrons in a molecule. It can be described as a property of an atom within a molecule. As helium is a noble gas it will not react to form molecules and will thus not be in a situation where it would attract a pair of electrons within a molecule.
Because it has never formed
There are, however, other electronegativity scales which do not depend on forming a homonuclear dimer, and
Other scales are shown here:
A more modern electronegativity chart is shown here.
Explanation:
By definition,
I think you meant to ask why helium has the HIGHEST ionization energy of any element...i.e. the energy required for the reaction...
...and such ionization reactions SPECIFY gaseous products and gaseous reactants. Now for helium, we gots a closed shell configuration...i.e. a nuclear charge of
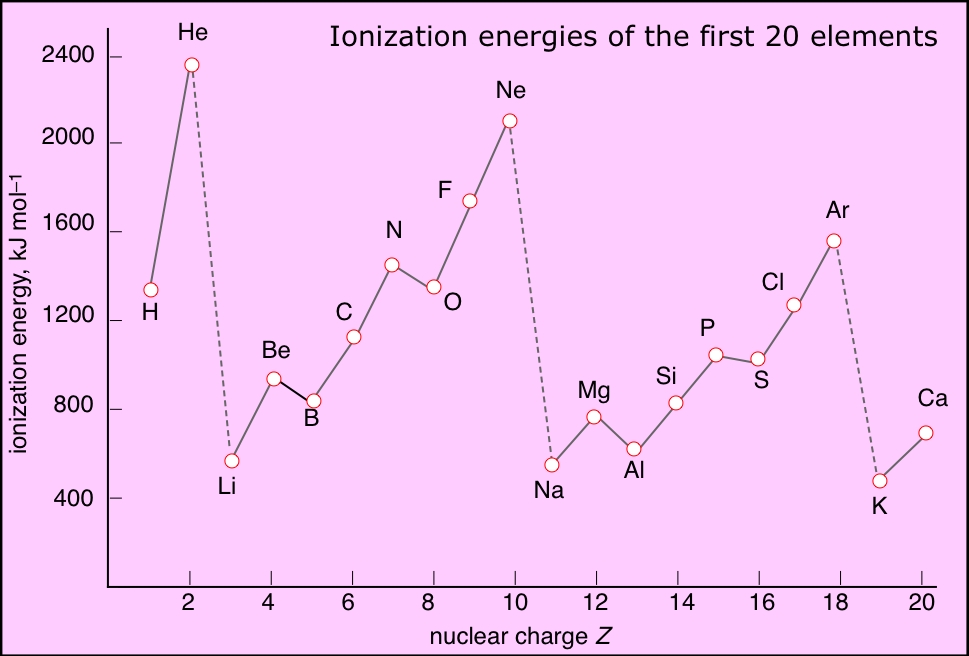
And thus helium has the highest ionization energy, and forms NO conventional compounds. Why are the ionization energies of each alkali metal so disproportionately low?



