Why is isopropyl alcohol used to zero the spectrophotometer?
1 Answer
I'm not sure where you got this question, but maybe something like here (pg. 9):
http://www.chem.purdue.edu/teacher/table_of_contents/uvvus/uvvis.plant%20pigments_ch.pdf
To zero the spectrophotometer is to set a standard for the blank spectrum.
The blank spectrum is like a "reference point" for actual sample spectra that you take. Some spectrophotometers let you subtract the blank spectrum from the sample spectrum so that you can remove stray light and other background interferences with the spectrum's accuracy to the sample itself.
This can be hard to understand without having done it yourself. I'm going to try to illustrate it with two
This is the
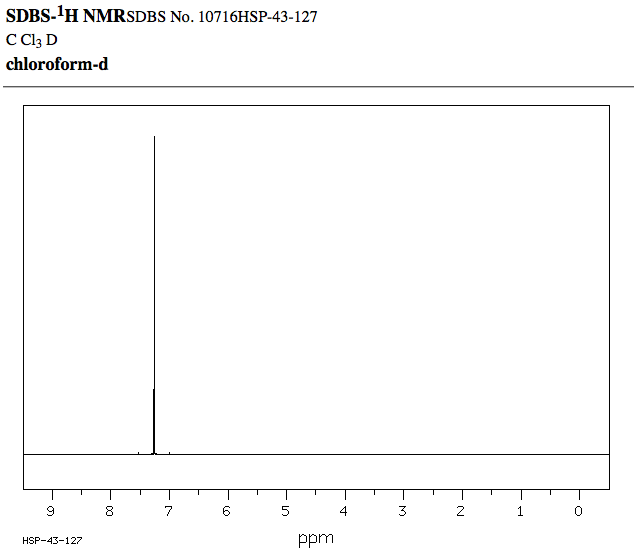
Notice how there are three peaks between
This is the
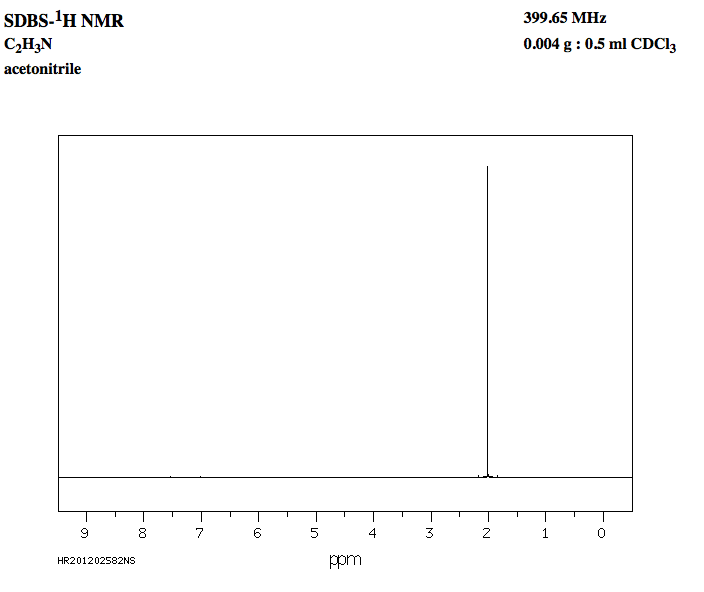
Notice how even though acetonitrile is in

