Because of the capacity for additional favorable #\mathbf(pi)# orbital overlap due to the substantial #\mathbf(pi)# electron density. You can read more about this here.
Maleic anhydride looks like this:
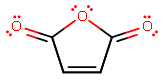
It has substantial #pi# electron density contribution from the nearby carbonyl carbons, which tends to increase the kinetic favorability in a Diels-Alder reaction, sometimes even with a seemingly sterically-hindered mechanism. It depends on the other reactant though.
One common lab that is done in organic chemistry 2 is the Diels-Alder reaction between maleic anhydride and cyclopentadiene.
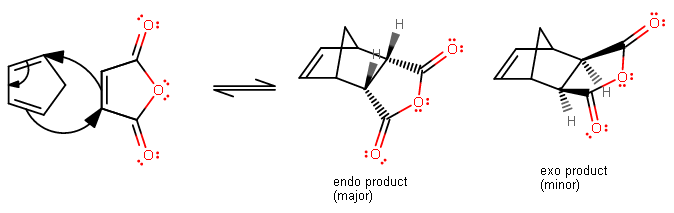
The orbital overlap during the reaction looks more like this:
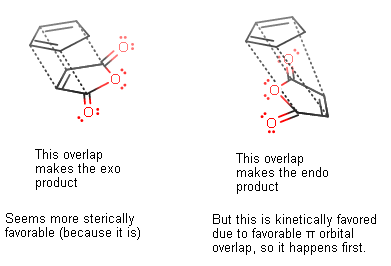
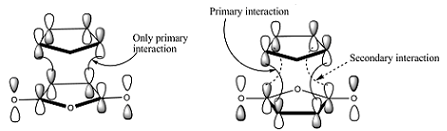
Normally one would expect the exo product because it has less steric interference, but because the "secondary" orbital overlap with the extra #pi# electron density from the carbonyl carbons makes the endo product the kinetically favorable product, even though the exo product is thermodynamically favorable, we get the endo product first. We don't see as much of the exo product.
In fact, according to the data I calculated for it while working with the computational software GAUSSIAN, the endo product is about #89.53%# favored and the exo product is about #10.47%# favored in this case. It's very significant!

