Why is neutralization not a redox reaction?
1 Answer
Apr 27, 2014
First, what is neutralization?
In chemistry, neutralization is a chemical reaction in which an acid and a base react to form a salt. Water is frequently, but not necessarily, produced as well.
But, what is redox reaction?
Redox (or reduction-oxidation) reactions include all chemical reactions in which atoms have their oxidation state changed; in general, redox reactions involve the transfer of electrons between species. For example:
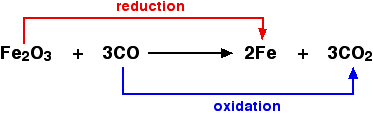
In neutralization there is no transfer of electrons between species or atom didn't change its oxidation state.

