Why is only one of the enantiomers of Ibuprofen effective?
1 Answer
Only one of the isomers is effective because it is the one that fits the receptor site on the enzyme involved in pain perception.
Explanation:
What ibuprofen does
Ibuprofen is works by inhibiting two enzymes called COX-1 and COX-2.
They convert arachidonic acid to prostaglandin H2 (PGH2) which, in turn, is converted by other enzymes to other prostaglandins that activate the body's response to inflammation.
How ibuprofen does it
Ibuprofen is 2-(4-isobutylphenyl)propanoic acid. Its structure is
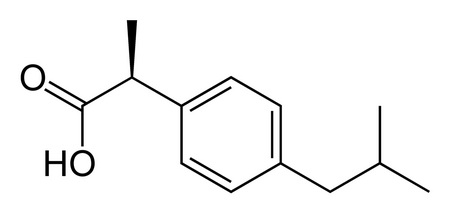 Ibuprofen
Ibuprofen
(From scienceline.ucsb.edu)
Note the chiral centre at
Why only the
The (
If we use the lock-and-key theory of enzyme action, we say that (
When it occupies the receptor site, it blocks access to the COX activators.
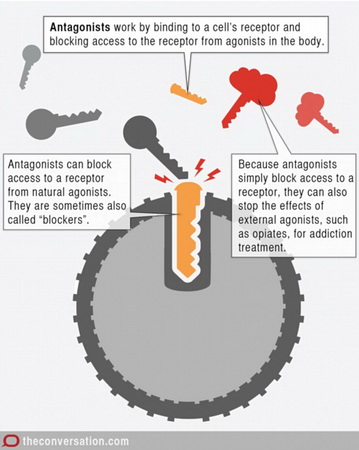 Lock and key
Lock and key
(From www.dailymail.co.uk)
The production of PGH2 ceases along with the pain and fever caused by the body's inflammatory response.

