Why is sulphuric acid needed as a catalyst in an esterification reaction?
1 Answer
Sulfuric acid provides the hydronium ions that protonate the carbonyl oxygen and make the carbonyl carbon a better electrophile.
Explanation:
Even if you are using concentrated sulfuric acid, it contains 2 % water, so hydronium ions are present:
The first step involves protonation of the carbonyl oxygen by the hydronium ion.
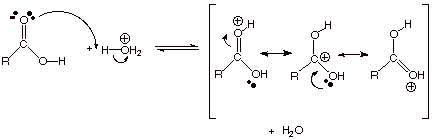
The product has three resonance contributors, and one of these puts a positive charge on the carbonyl carbon atom.
This makes the carbon much more electrophilic and susceptible to attack by the oxygen of the alcohol in the second (and rate-determining) step.
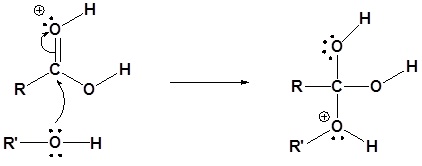
Without this enhanced electrophilicity, the activation energy for nucleophilic attack would be much higher, and the rate of reaction would be much slower.
Hence, the sulfuric acid is acting as a catalyst for the reaction: it provides an alternate pathway that has a lower activation energy.

