Why is combustion an exothermic reaction?
1 Answer
Combustion reaction produce products which have a lower energy state than the reactants which were present before the reaction.
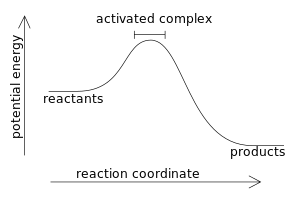
A fuel (sugar for example) has a great deal of chemical potential energy. When the sugar burns by reacting with oxygen, it produces mostly water and carbon dioxide. Both water and carbon dioxide are molecules which have less stored energy than what sugar molecules have.
Here is a video which discusses how to calculate the enthalpy change when 0.13g of butane is burned.
Video from: Noel Pauller
Here is a video which shows the combustion of sugar. The reaction goes much more rapidly than normal because it is aided by the use of potassium chlorate (an oxidizing agent used in fireworks).
Video from: Noel Pauller
Hope this helps!

