Why is there are huge energy change between the 9th and the 10th electron in the sodium atom?
the text book that I was reading says that "the large jump from the ninth to the tenth electron indicates another significant change in energy of the electron. This corresponds to an electron being removed from the first quantum shell: the 1s orbital. THe tenth ionisation energy is larger than the ninth, since as one electron is removed from the 1s orbital, the remaining electron now experiences zero repulsion, so its energy decreases" do they mean that the ionisation energy of the 10th electron is larger because it is located in the 1st quantum shell and therefore it requires high energy to be removed? 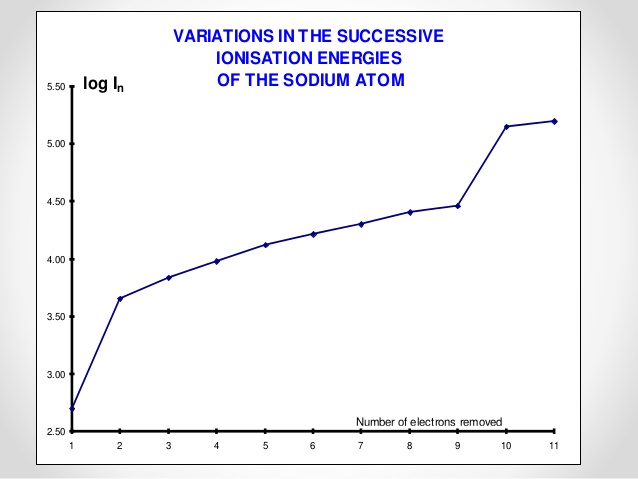
the text book that I was reading says that "the large jump from the ninth to the tenth electron indicates another significant change in energy of the electron. This corresponds to an electron being removed from the first quantum shell: the 1s orbital. THe tenth ionisation energy is larger than the ninth, since as one electron is removed from the 1s orbital, the remaining electron now experiences zero repulsion, so its energy decreases" do they mean that the ionisation energy of the 10th electron is larger because it is located in the 1st quantum shell and therefore it requires high energy to be removed? 
1 Answer
A change in principal quantum number (shell) means a larger jump in ionisation energy, compared to ionisation energy changes within the same shell. Between the ninth and tenth ionisation energies you are going from principal quantum number 2 to 1, so there is a larger energy difference.
Explanation:
The bold sentence should read:
The eleventh ionisation energy is larger than the tenth, since as one electron is removed from the 1s orbital, the remaining electron now experiences zero repulsion, so its energy decreases and it's ionisation energy increases.
The electron configuration for a sodium atom is
Here is it's representation showing the energies of each orbital.
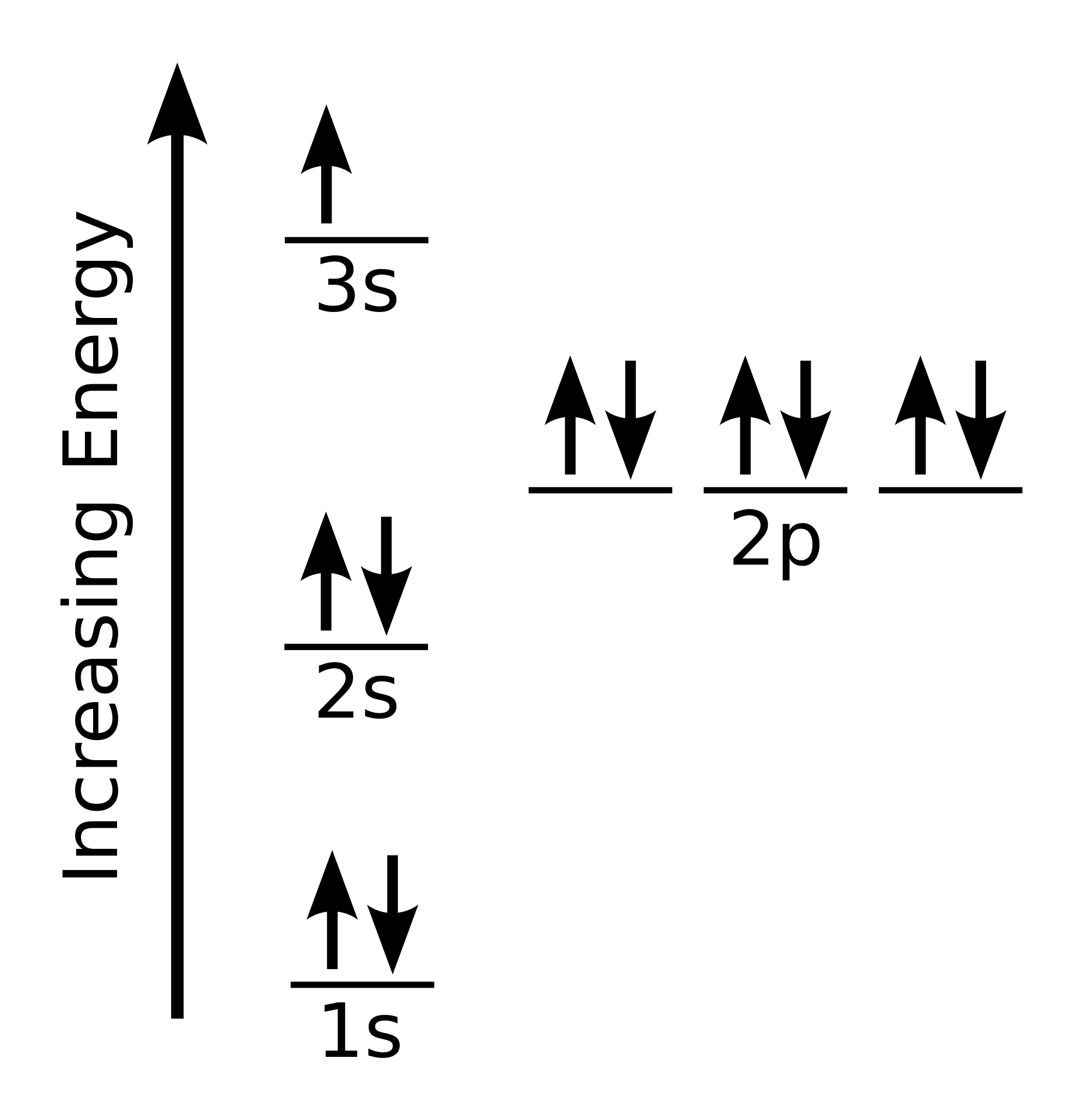
Remember that the larger the principal quantum number (shell number), the higher the potential energy of the orbital. The higher the potential energy of the orbital, the lower the ionisation energy for an electron in that orbital. For example, the ionisation energy for the 3s electron is the smallest and the 1s electrons have the largest ionisation energies.
Every time you change principal quantum number, you will see a bigger increase in ionisation energy, due to the energy gap between shells being bigger than the energy gap between electrons in a particular shell. I.e. All the electrons in the second quantum shell are closer in energy to each other than they are to the electrons in the first shell.
Counting from the highest energy electron first (3s), the eighth and ninth electrons correspond to 2s electrons. The tenth and eleventh electrons are the 1s electrons.
So, from first to second ionisation energies you see a big jump corresponding to principal quantum number change of 3 to 2. Also, from ninth to tenth ionisation energies, the principal quantum number goes from 2 to 1, so there is another big jump.

