Why is water considered to be electronegative?
1 Answer
It's not. Molecules are never considered electronegative. Electronegativity is attributed to atoms, and atoms only. Polarity is attributed to molecules.
Water is polar because the electronegativity of oxygen (
Thus, more negative charges are near oxygen and more positive charges are near the hydrogens.
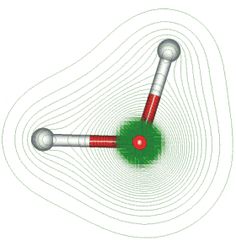
Another way to say it is that the dipole moment vector is pointed through the


