Write the equilibrium constant expression for the following reactions?
Write the equilibrium constant expression for the following reactions? 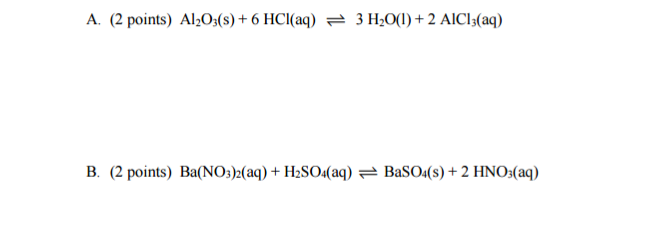
Write the equilibrium constant expression for the following reactions? 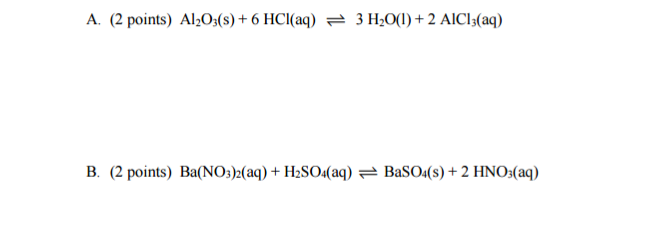
1 Answer
A few principles to keep in mind:
- Products over reactants for the forward reaction, and square brackets for concentration of aqueous species.
- Coefficients go into the exponents of the substance concentration.
- Pure liquids and solids are written as
#1# in the#K# mass action expression.
As a result,
#color(blue)(K_c = (["AlCl"_3]^2)/(["HCl"]^6))# Fairly straightforward; the solid and liquid are implied to be
#1# , and hence are invisible. I didn't write a net ionic reaction for this one, but did for#(B)# , because#(A)# has#"AlCl"_3# , which is not quite ionic and not quite covalent.
#K_c = (["HNO"_3]^2)/(["Ba"("NO"_3)_2]["H"_2"SO"_4])# This second one looks rather clunky though, given that nitrates are generally quite soluble, and nitric acid is a strong acid... I would have rewritten this as its net ionic reaction:
#"Ba"^(2+)(aq) + "SO"_4^(2-)(aq) rightleftharpoons "BaSO"_4(s)# which is the precipitation equilibrium to form barium sulfate solid.
This has
#color(blue)(K_(sp)^(-1)) = 1/(["Ba"^(2+)]["SO"_4^(2-)]) = color(blue)(["Ba"^(2+)]^(-1)["SO"_4^(2-)]^(-1))#

