What is the bond polarity of h2o?
1 Answer
Bond polarity refers to the separation of charge within a bond.
Bonds between atoms of different electronegativity are polar. The more electronegative atom has a higher density of bonding electrons around it, giving it a partial negative charge (δ⁻). The less electronegative atom has some of its electron density taken away, giving it a partial positive charge (δ⁺).
In water, the separation of charge in the O-H bonds is due to the different electronegativities of oxygen and hydrogen. Since oxygen is more electronegative than hydrogen, an O-H bond is polar covalent. Each O-H bond is polar covalent with a partial negative charge on the oxygen atom.
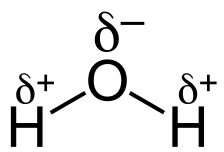
The polarity in the bonds is also shown by an arrow representing a dipole (two charges separated by a distance). There is a + sign at the tail of the arrow. The head of the arrow points the + sign towards the δ⁻ atom. Thus, water has two O-H bond dipoles, each of which points towards the O atom.


