How do you sketch galvanic cells?
1 Answer
You draw the anode, cathode, the oxidizing and reducing agents, a porous plate or a salt bridge. You show the direction of electron flow in the external circuit and the flow of ions in the internal circuit.
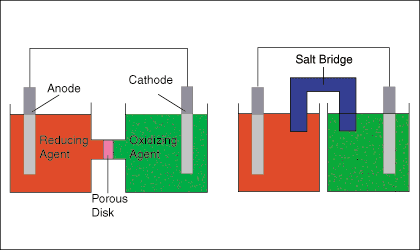
EXAMPLE:
Sketch a cell diagram for the reaction
Zn(s) + Cu²⁺(aq) → Zn²⁺(aq) + Cu(s)
Solution:
A. Draw one of the diagrams above (no labels).
B. Identify what is oxidized and reduced.
Zn is oxidized; Cu²⁺ is reduced.
C. Put the oxidation materials in the left hand cell. Label the Zn as the negative anode. In the solution put Zn²⁺ and NO₃⁻.
D. Put the reduction half-reaction materials in the right hand cell. Label the Cu as the positive cathode. In the solution put Cu²⁺ and either SO₄²⁻ or NO₃⁻.
E. Place a salt such as KNO₃ in the salt bridge.
F. Use arrows to show the flow of electrons in the external wire.

G. Show the flow of ions in the internal circuit.
The cations — Zn²⁺, Na⁺, and Cu²⁺ — move from left to right. The NO₃⁻ anions move from right to left. Draw arrows to show these motions.

